2025 Vol. 7, No. 39
Rabies is a zoonotic disease caused by rabies viruses(RABV). China is a high-risk country for rabies. To address China’s rabies situation, the Chinese Ministry of Agriculture and Rural Affairs issued the National Animal Disease Surveillance and Epidemiological Investigation Plan. This study systematically summarized animal rabies surveillance data from the past two decades based on the Program.
Suspected rabies cases collected through the Program between 2004 and 2024 underwent confirmatory diagnosis at the National Reference Laboratory (NRL) for animal rabies using national standard protocols: direct fluorescent antibody testing (FAT) and real-time RT-PCR. Epidemiological data from confirmed cases were analyzed using Geographic Information System (GIS) mapping and statistical evaluation methods.
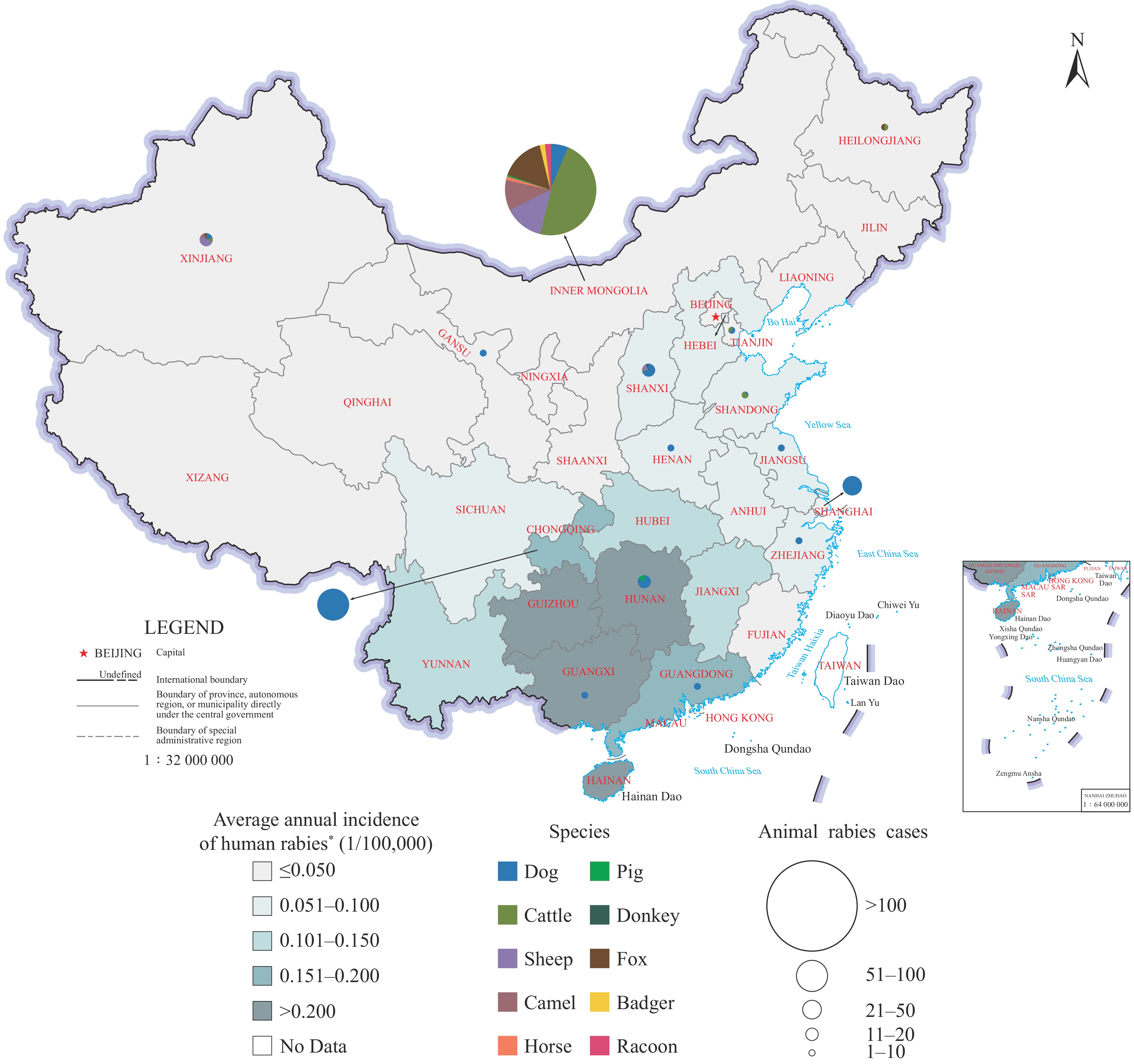
Laboratory diagnosis confirmed 331 of 433 suspected cases (76.44%) as rabies-positive. These confirmed cases originated from 15 provincial-level administrative divisions (PLADs) and revealed two distinct transmission patterns: a) dog-mediated rabies, accounting for 47.13% of cases and predominantly endemic in southern PLADs, where it poses ongoing human exposure risks; and b) wildlife-mediated rabies in livestock, comprising 52.87% of cases and primarily transmitted by foxes in northern PLADs, with the Inner Mongolia Autonomous Region (IMAR) experiencing the highest burden.
This nationwide surveillance has elucidated current rabies transmission dynamics across China, revealing persistent threats from dog rabies to human health in southern PLADs and emerging threats from wildlife-mediated rabies to livestock in northern border regions. These findings underscore the critical need for enhanced surveillance systems and targeted vaccination strategies addressing both domestic dog populations and wildlife reservoirs to achieve effective rabies control.
Rabies is a fatal, but preventable, viral disease. Post-exposure prophylaxis (PEP), which includes the administration of passive immune preparations, is critical after exposure to the rabies virus, particularly in high-risk cases. The delayed or missed application of passive immunizing agents may increase the risk of infection.
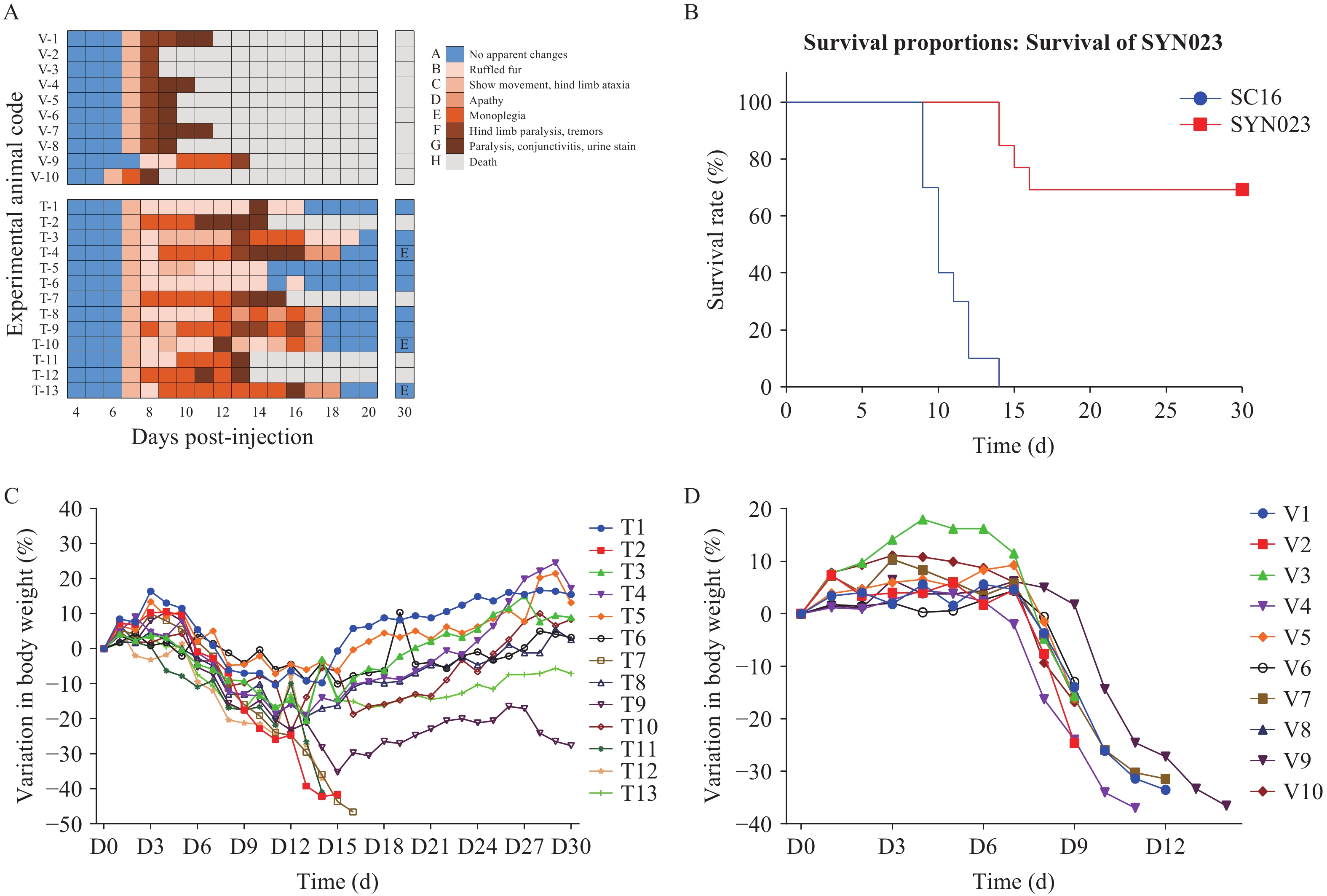
SYN023, a novel anti-rabies monoclonal antibody cocktail, can effectively reverse the course of rabies infection, even at a late stage of the disease. In a mouse model infected with the rabies virus strain SC16, multiple high-dose injections of SYN023 administered 5 days post-inoculation rescued 69% of the animals.
These findings suggest that SYN023 could serve as a promising therapeutic agent for rabies PEP, particularly in cases in which treatment initiation is delayed. This study provides a scientific basis for future clinical trials aimed at improving rabies treatment protocols.
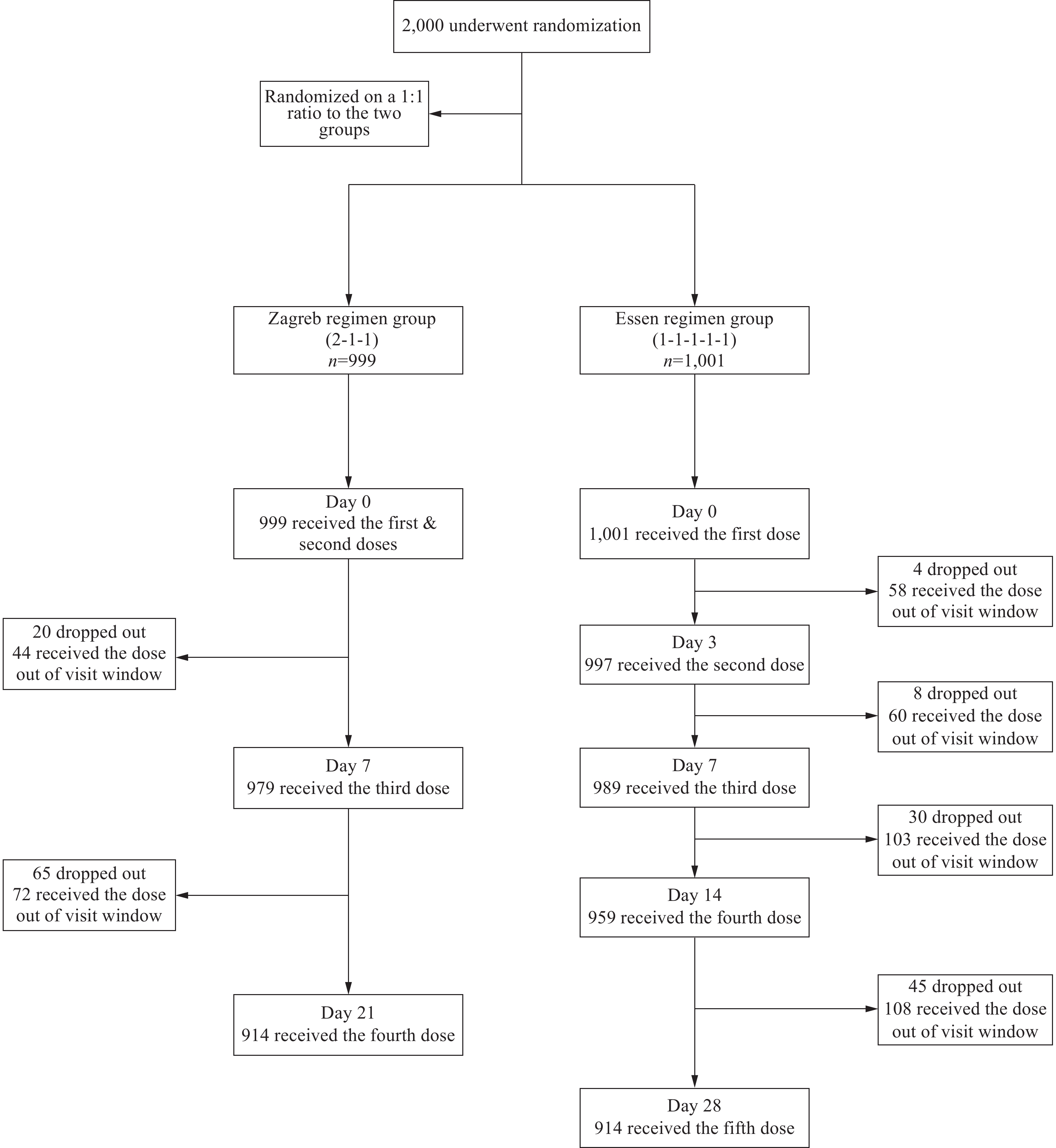
Both the 4-dose (Zagreb) and 5-dose (Essen) rabies vaccination regimens demonstrate comparable immunogenicity and safety profiles in clinical trials and are approved for use in China.
This study pioneers active safety surveillance via a mobile application, identifying an adverse reaction rate of 2.10% for the Zagreb regimen and 2.70% for the Essen regimen. The Zagreb regimen had a lower out-of-window administration rate of 8.41% compared to 16.38%. Compliance was influenced by age, marital status, and exposure level, while the Essen regimen involved additional factors, including education level and perceived convenience.
This study supports broader adoption of the 4-dose regimen to reduce logistical challenges and enhance compliance. Mobile application-based active surveillance presents a novel approach to enhancing real-world monitoring quality.
Human rabies remains nearly universally fatal despite medical advances. Diagnosis is frequently delayed when patients present with atypical symptoms, and the failure to receive postexposure prophylaxis (PEP) continues to be a major contributor to mortality worldwide.
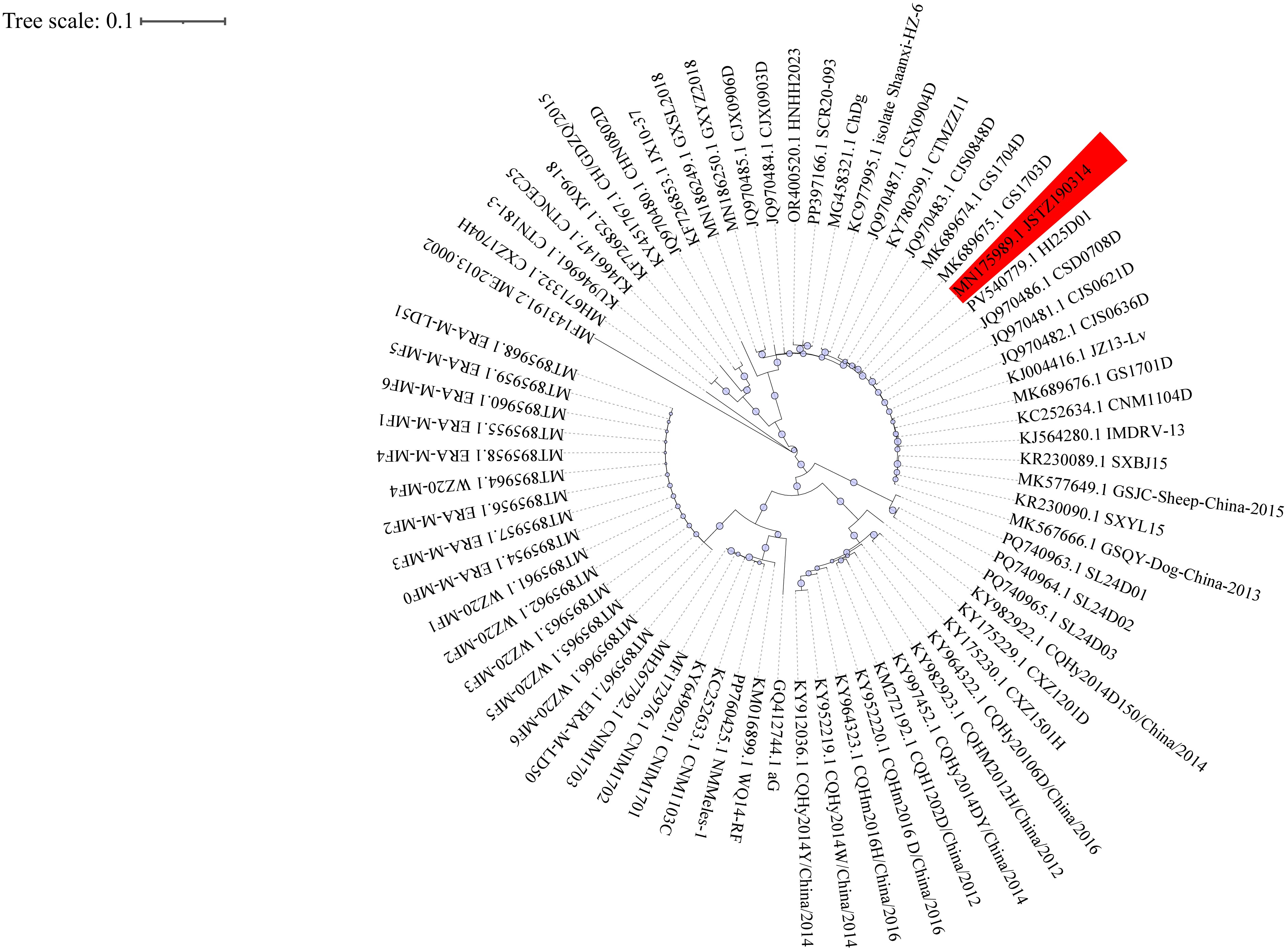
This represents the first confirmed human rabies case in Guangxi caused by the JSTZ190314 strain, successfully identified through metagenomic next-generation sequencing (mNGS). The patient initially presented with urinary symptoms that led to a misdiagnosis before characteristic neurological manifestations developed, ultimately progressing to brain death 28 days after neurological onset (34 days from initial urinary symptoms).
This case demonstrates the critical importance of mNGS in diagnosing atypical rabies presentations and emphasizes the urgent need for enhanced early clinical recognition, standardized PEP administration protocols, and strengthened regional viral surveillance systems.
Post-exposure prophylaxis (PEP) represents the critical intervention for preventing rabies and comprises three essential components: thorough wound cleansing, vaccination, and administration of rabies immunoglobulin (RIG) or monoclonal antibodies (mAbs) for category III exposures. The World Health Organization (WHO) endorses the use of mAb cocktails as an effective replacement for RIG in PEP protocols. Since 2016, four anti-rabies monoclonal antibodies (RmAbs) have received clinical approval for use in India and China. This article provides an overview of the current research status of RmAb. By reviewing clinical studies related to RmAb, it highlights the clinical advantages of RmAb over HRIG in terms of efficacy, accessibility, safety, acceptability, and clinical application in special populations. Additionally, it explores the future clinical prospects of RmAb, including their use in extremely high-risk cases, their impact on circulating antibodies, and their potential role in rabies treatment.



 Subscribe for E-mail Alerts
Subscribe for E-mail Alerts CCDC Weekly RSS Feed
CCDC Weekly RSS Feed