-
Introduction: This study reports a confirmed case of human rabies diagnosed through metagenomic next-generation sequencing (mNGS). The patient was a 22-year-old female who developed symptoms 3 months after sustaining a scratch on the upper lip from a domesticated dog, without receiving postexposure prophylaxis (PEP). She initially presented with urinary symptoms and was misdiagnosed with a urinary tract infection. Neurological symptoms subsequently emerged, prompting intensive life support interventions including mechanical ventilation, recombinant human interferon-α2b, ribavirin, norepinephrine, veno-arterial extracorporeal membrane oxygenation (VA-ECMO), and continuous renal replacement therapy.
Methods: Clinical data were collected from hospital records, including exposure history, symptoms, treatments, and outcomes. Saliva specimens were tested by reverse transcription polymerase chain reaction (RT-PCR) and mNGS. Sequencing data were processed by standard bioinformatics pipelines, and phylogenetic analysis was performed with MAFFT alignment and IQ-TREE maximum-likelihood reconstruction.
Results: Rabies virus infection was confirmed through reverse transcription polymerase chain reaction (RT-PCR) and mNGS analysis of saliva samples. The detected strain, JSTZ190314, represents the first documented case of this genotype in Guangxi, China. Despite initial stabilization with ECMO support, the patient’s neurological condition deteriorated progressively, leading to brain death 28 days after neurological onset (34 days from initial urinary symptoms).
Conclusion: mNGS proves invaluable as a diagnostic tool for atypical rabies presentations. Enhancing early clinical recognition capabilities, ensuring timely and standardized PEP implementation, and strengthening regional viral strain surveillance represent critical components for effective rabies prevention and control strategies.
-
Rabies represents a fatal zoonotic disease caused by the rabies virus (RABV), resulting in over 59,000 human deaths globally each year, with the majority of cases occurring in Asia and Africa (1-2). Early diagnosis of rabies remains challenging due to the low detection rates of viral RNA in saliva and cerebrospinal fluid (CSF) during the initial stages of infection (3). Although the direct fluorescent antibody (DFA) test continues to serve as the criterion standard for rabies diagnosis, its sensitivity depends heavily on specimen quality, operator experience, and laboratory conditions. This variable sensitivity renders the DFA test susceptible to false-negative results, particularly in patients with low viral loads or early-stage infections. Similarly, polymerase chain reaction (PCR) methods have enhanced detection capabilities but are not without limitations, particularly in resource-constrained settings where accessibility and reproducibility may be compromised (4–5).
More recently, metagenomic next-generation sequencing has emerged as a powerful, unbiased, high-throughput tool for the rapid identification of infectious pathogens, particularly in cases involving rare, atypical, or difficult-to-detect organisms (6–7). Here, we report a patient with a rabies infection who survived 28 days from neurological onset (34 days from initial urinary symptoms) caused by the JSTZ190314 strain — the first such case in Guangxi Zhuang Autonomous Region, China — as confirmed via metagenomic next-generation sequencing. This report aims to highlight the diagnostic value of metagenomic next-generation sequencing in early rabies detection, describe the clinical course under aggressive supportive care, and explore the public health implications of a newly identified RABV strain.
Clinical data were obtained from hospital medical records, including demographics, exposure history, clinical manifestations, laboratory findings, imaging, and therapeutic interventions.
Saliva samples were tested for rabies virus RNA using reverse transcription polymerase chain reaction (RT-PCR). Metagenomic next-generation sequencing (mNGS) was performed on the GeneMind FASTASeq 300 platform (single-end 75 bp, >20 million reads per sample, Q30 ≥85%). Data were processed with fastp for quality control, low-complexity reads were filtered with PRINSEQ, and host sequences were removed by alignment to the hg38 human reference genome using BWA-MEM. Non-host reads were taxonomically classified with Kraken2 and validated by re-alignment.
Complete N gene sequences were aligned using MAFFT v7.490 (--auto), and phylogenetic reconstruction was conducted with IQ-TREE v2.0.7 (GTR+G model, 1,000 ultrafast bootstrap replicates). Visualization was performed with iTOL v7.2.1.
A previously healthy 22-year-old woman developed symptoms 3 months after sustaining a scratch on her upper lip from a domesticated dog in September 2024. Although the wound bled significantly, she only rinsed it with tap water and received neither rabies vaccination nor passive immunization.
On December 22, 2024, she experienced acute urinary frequency, urgency, and vulvar burning. Three days later, she visited a local hospital, where clinicians initially diagnosed urinary tract infection and vulvitis. Despite empirical antimicrobial therapy and symptomatic treatment, her condition showed no improvement. On December 28, the patient developed fever (38.2 °C), chills, pharyngeal spasms, muscle twitching, dysphagia, and pronounced anxiety. She presented to our emergency department where rabies was suspected, prompting immediate admission to the intensive care unit due to hemodynamic instability.
Upon admission, the patient exhibited agitation, hypersalivation, and an exaggerated pharyngeal reflex. She demonstrated marked hydrophobia and aerophobia with extreme sensitivity to auditory and airflow stimuli. Her vital signs revealed instability: heart rate 145 beats/min, blood pressure 90/50 mmHg, and peripheral oxygen saturation (SpO2) of 88% on room air. Laboratory findings showed leukocytosis (15.8×109/L) and elevated myocardial enzymes [creatine kinase (CK)-MB: 250.1 U/L; troponin T: 0.487 ng/mL]. Echocardiography revealed globally reduced cardiac systolic function with a left ventricular ejection fraction (LVEF) of 16%, suggesting concurrent viral myocarditis.
Due to rapid neurologic deterioration and circulatory collapse, the patient underwent emergency endotracheal intubation and mechanical ventilation on December 28. The following day, she progressed to cardiogenic shock despite high-dose norepinephrine infusion [3.5 μg/(kg·min)]. We urgently initiated veno-arterial extracorporeal membrane oxygenation (VA-ECMO) and continuous renal replacement therapy (CRRT) to stabilize vital signs. Concurrently, we administered antiviral therapy with ribavirin and recombinant interferon-α2b, along with corticosteroids, intravenous immunoglobulin, and comprehensive supportive care.
Following standard laboratory protocols for rabies diagnosis, we initially planned to collect multiple saliva specimens for testing. However, the first specimen collected on January 3, 2025, already tested positive for rabies virus RNA by RT-PCR (Ct=26.38). Given the concordant clinical presentation, we submitted this same sample for metagenomic next-generation sequencing (mNGS) and did not pursue additional specimen collection. On January 5, mNGS confirmed the presence of RABV infection. We performed sequencing on the GeneMind FASTASeq 300 platform using single-end 75 bp reads, generating over 20 million raw reads per sample with Q30 scores ≥85%. After quality control with fastp and removal of low-complexity sequences, we aligned clean reads to the human reference genome (hg38) using BWA-MEM and excluded host reads. We then taxonomically classified non-host reads with Kraken2 and validated results by re-alignment to reference genomes. To filter potential contaminants, we used negative controls and a laboratory background database, considering only microbes with reads per million (RPM) ≥3× negative template control (NTC) as positive. We annotated antimicrobial resistance and virulence genes by mapping against the CARD and VFDB databases. Sequence alignment identified the strain as JSTZ190314, marking the first reported case of this genotype in Guangxi, China. The evolutionary relationship of the patient-derived strain is shown in Figure 1.
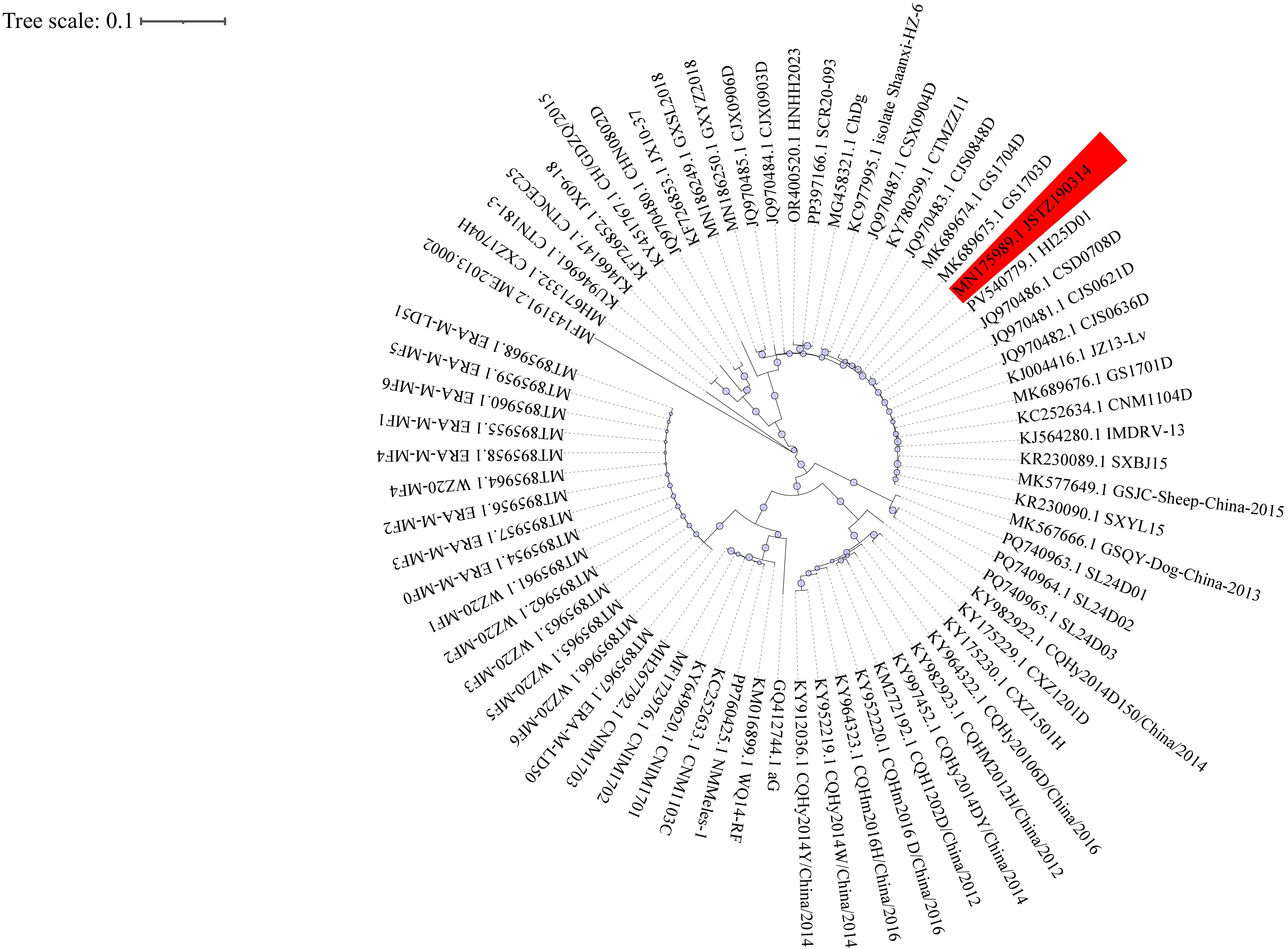 Figure 1.
Figure 1.Phylogenetic analysis of Rabies lyssavirus strains in China.
Note: Circular maximum-likelihood phylogenetic tree of 70 complete N gene sequences of Rabies lyssavirus strains circulating in China. The tree was reconstructed using IQ-TREE v2.0.7with themaximum-likelihood methodunder theGTR+G substitution modeland1000 bootstrap replicates, based on multiple-sequence alignment performed by MAFFT v7.490 (–auto option). Bootstrap values (≥70) are shown at major nodes. The patient-derived strain JSTZ190314 is highlighted, with host species and geographic origins annotated from GenBank records. Visualization was performed with iTOL v7.2.1. Abbreviation: MAFFT=Multiple Alignment using Fast Fourier Transform; IQ-TREE=software for phylogenetic inference using maximum likelihood.The patient was successfully weaned off ECMO on January 7, but her neurological condition progressively deteriorated. She remained in a deep coma with no spontaneous respiration, bilaterally dilated and fixed pupils, and absent pupillary light reflexes. Neurological evaluation confirmed brain death. Following written informed consent from the patient’s legal guardian, we withdrew life-sustaining treatment on January 25, and the patient was declared clinically dead. The patient progressed to brain death 28 days after neurological symptom onset (34 days from initial urinary symptoms). Figure 2 summarizes the clinical course.
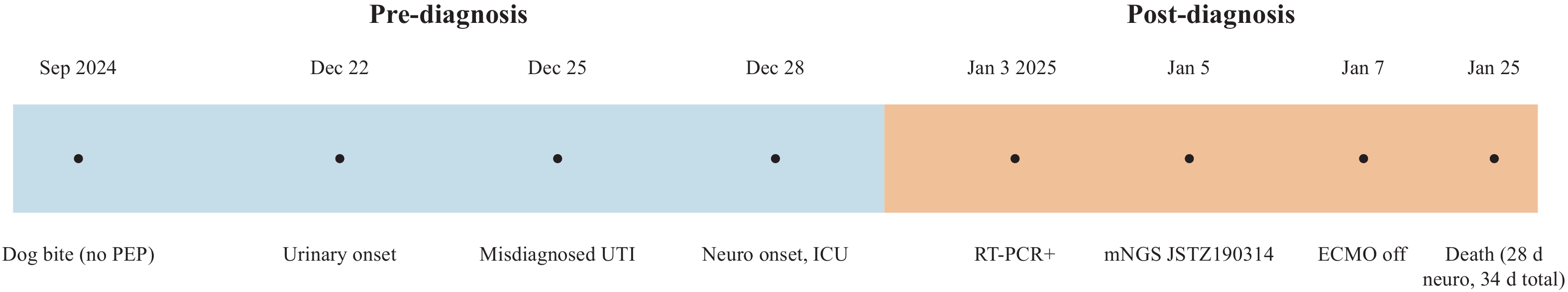 Figure 2.
Figure 2.Clinical timeline of the reported rabies case.
Note: Pre-diagnosis events (blue): dog bite without PEP, urinary onset, misdiagnosis as UTI, and neurological onset with ICU admission. Post-diagnosis events (orange): saliva RT-PCR positive, mNGS confirmation of strain JSTZ190314, ECMO weaning, and death 28 days after neurological onset (34 days from initial symptoms).
Abbreviation: PEP=postexposure prophylaxis; UTI=urinary tract infection; mNGS=metagenomic next-generation sequencing; ECMO=extracorporeal membrane oxygenation.
-
Rabies represents a nearly universally fatal viral infection of the central nervous system, with patients typically surviving seven to ten days following neurological symptom onset (8). This case involved a previously healthy 22-year-old woman who developed rabies three months after sustaining a dog scratch without receiving postexposure prophylaxis (PEP). Remarkably, her clinical course extended 28 days from neurological symptom onset (34 days from initial urinary symptoms), substantially exceeding typical survival duration and potentially reflecting temporary stabilization achieved through intensive life support interventions.
The patient’s initial presentation with urinary symptoms — including dysuria, frequency, urgency, and vulvar burning — led to misdiagnosis as a genitourinary infection. Rabies was only suspected after classic neurological manifestations emerged, including aerophobia, dysphagia, and pharyngeal spasms. This clinical trajectory highlights the diagnostic challenges posed by atypical early rabies presentations and reveals critical gaps in exposure history assessment and public awareness regarding dog-related injury risks.
Traditional diagnostic approaches, including the DFA test and standard RT-PCR, demonstrate reduced sensitivity when viral loads are low or sample types are limited, frequently resulting in delayed diagnosis of rare or atypical pathogens (9-10). In this case, rabies virus RNA was initially detected in saliva samples through RT-PCR and subsequently confirmed via metagenomic next-generation sequencing, which identified the infecting strain as JSTZ190314. As an unbiased, high-throughput diagnostic method requiring no prior pathogen knowledge, mNGS demonstrates considerable promise for both public health emergencies and complex infectious disease diagnosis. However, mNGS faces significant limitations, including resource intensity, high costs, and requirements for advanced laboratory infrastructure and bioinformatics expertise. Additionally, false-positive results may occur due to sample contamination or sequencing artifacts, necessitating careful interpretation alongside clinical findings and confirmatory testing. These constraints may limit routine implementation in resource-limited or primary care settings.
To our knowledge, the JSTZ190314 strain identified in this case represents the first documented human infection with this genotype in Guangxi. Phylogenetic analysis was performed using MAFFT v7.490 (--auto) for sequence alignment, followed by reconstruction in IQ-TREE v2.0.7 under the GTR+G model with 1,000 ultrafast bootstrap replicates. This analysis confirmed that JSTZ190314 belongs to the Asian lineage and demonstrates high sequence identity with previously reported canine-origin rabies virus strains. The phylogenetic tree (Figure 1) illustrates the evolutionary placement of JSTZ190314 within the Asian lineage of Rabies lyssavirus strains circulating in China. Originally isolated in 2019 by Cheng et al. from a bovine case in eastern China, phylogenetic analysis confirmed its classification within the Asian lineage of canine origin (11). The detection of this strain in a human patient indicates an unrecognized transmission pathway in the region. In Guangxi, domestic dogs serve as the primary reservoir for human rabies transmission, though stray cats and bats may also contribute to viral maintenance and circulation. Given the patient’s semi-rural residence and documented dog-bite exposure, local canine populations represent the most probable source of infection. However, domestic dogs in such environments frequently interact with stray cats through feeding behaviors or territorial disputes, and occasionally encounter bats through predation, creating potential pathways for cross-species viral spillover. These findings highlight the critical need for enhanced molecular surveillance of dogs, stray animals, and wildlife populations within a comprehensive One Health framework. Such surveillance should include molecular tracing of circulating viral strains and potential updates to the protective spectrum of current rabies vaccines to ensure continued efficacy against emerging variants.
Following rabies confirmation, the patient received comprehensive life support interventions, including endotracheal intubation, mechanical ventilation, VA-ECMO, and CRRT. Although the patient ultimately progressed to brain death, her survival duration exceeded the typical median survival time of 7–10 days after symptom onset in rabies patients (12). This extended survival may reflect temporary stabilization of multiorgan dysfunction under intensive care, though causality cannot be established from a single case with multiple concurrent interventions. While ECMO likely contributed to maintaining cardiopulmonary function, other supportive measures — including CRRT, corticosteroids, and immunoglobulin therapy — may have provided synergistic benefits. The independent contribution of ECMO cannot be definitively isolated without comparative cohort data or multifactorial analysis. Kuang et al. reported a similar case diagnosed via both NGS and PCR, where the patient experienced temporary stabilization of multiorgan dysfunction under intensive support (13). Although no antiviral agent has demonstrated definitive efficacy against rabies, the patient received ribavirin and recombinant human interferon-α2b based on empirical treatment strategies reported in the literature, including components of the Milwaukee protocol. These agents were administered under compassionate care principles given the absence of established alternatives. Their use required careful monitoring for potential adverse effects, including hematologic suppression and hepatic dysfunction. These cases suggest that life-sustaining measures such as ECMO and CRRT may provide temporary supportive bridges in selected patients, allowing time for virological confirmation and family communication. However, the clinical impact of such interventions on overall outcomes remains uncertain and requires further investigation. In resource-adequate settings, aggressive supportive therapy may therefore warrant additional evaluation as a component of rabies care for selected patients.
This report has several limitations, including the absence of pathological or postmortem confirmation through brain tissue immunofluorescence or virus isolation, which was not performed due to family refusal of autopsy. Additionally, sample collection was limited in both type and timing, and systematic multi-specimen testing was not conducted. Nevertheless, the diagnosis was robustly supported by characteristic clinical features, RT-PCR results, and mNGS confirmation.
Given the near-universal fatality of rabies after symptom onset (14), timely and standardized postexposure prophylaxis remains the cornerstone of prevention. This prophylaxis includes immediate and thorough wound cleansing, timely administration of rabies vaccines, and appropriate use of passive immunization agents (15–16). However, public awareness remains insufficient, and inconsistencies in the management of animal exposures, particularly in rural and resource-limited areas, continue to pose serious challenges to the effective implementation of PEP (17–18). Therefore, enhancing health education and capacity-building among community residents and primary healthcare providers is essential for improving prevention strategies and reducing rabies-related mortality (19–21).
Atypical early manifestations of rabies can lead to misdiagnosis and delayed diagnosis. In this case, rabies virus infection was confirmed by mNGS, with the first identification of the JSTZ190314 strain in Guangxi. The patient progressed to brain death 28 days after neurological onset (34 days from initial urinary symptoms), longer than typically reported, possibly reflecting temporary stabilization under intensive care; however, causality cannot be inferred. These findings emphasize mNGS as a valuable diagnostic tool and reinforce that timely, standardized PEP remains the cornerstone of rabies prevention.
-
The GXMU REXPLO MEDICAL LAB for providing technical assistance with metagenomic next-generation sequencing (mNGS) analysis. We also acknowledge Robin James Storer, PhD, from Liwen Bianji (Edanz) (www.liwenbianji.cn) for his editorial assistance with the English text of this manuscript.
-
Approval from the Ethics Committee of the International Zhuang Medicine Hospital Affiliated to Guangxi University of Chinese Medicine (approval No. 202415802). Written informed consent for publication, including authorization to use clinical information and images, was obtained from the patient’s legal guardian. All procedures adhered to the ethical standards established by our institutional and national research committees and complied with the 1964 Helsinki Declaration and its subsequent amendments (2013), along with comparable ethical standards.
HTML
| Citation: |



 Download:
Download:




