-
Introduction: Rabies is a lethal yet preventable viral disease caused by lyssaviruses. It is recommended that post-exposure prophylaxis (PEP) be administered as early as possible after viral exposure. The application of passive rabies immune preparations is an important measure of rabies PEP, especially for high-risk exposures. However, owing to various factors, patients exposed to the rabies virus may not receive passive immune agents in a timely manner, thereby increasing the risk of infection.
Methods: We evaluated the clearance efficacy of SYN023, an anti-rabies cocktail monoclonal antibody (mAb), in mice infected with the rabies virus strain SC16.
Results: Our results demonstrated that SYN023 rescued 69% of the infected mice at a late stage (5 days post-inoculation).
Conclusion: Multiple daily injections of high-dose SYN023 reversed rabies infection during the late exposure stage. These findings provide a critical scientific groundwork to guide the design and implementation of future clinical trials for rabies therapy.
-
Rabies is a zoonotic infectious disease characterized by acute progressive encephalomyelitis caused by lyssaviruses, including the rabies virus (RABV). Despite its nearly 100% fatality rate, timely and comprehensive post-exposure prophylaxis (PEP) remains an effective preventive measure. Following rabies exposure, in addition to thorough wound cleaning and vaccination, passive immunization preparations are administered as required. The WHO Expert Advisory Panel on Rabies recommends that for individuals exposed to Category III RABV, vaccination should be accompanied by meticulous wound cleaning and administration of passive immune agents, such as human rabies immunoglobulin (HRIG), equine rabies immunoglobulin (ERIG), or rabies monoclonal antibody (mAb) to prevent viral entry into the nervous tissue and provide immediate protection (1).
Owing to advantages such as standardized production and higher neutralizing titers compared to polyclonal immunoglobulin preparations, mAbs represent a promising alternative to HRIG and ERIG (2). Notably, mAbs can easily reach a much higher neutralization titer against RABV than HRIG, allowing them to neutralize the RABV that has already been systematically exposed (3). In 2020, in a groundbreaking study, mice were challenged with the RABV and administered a combination of rabies mAbs (RVC20 and RVC58) via peripheral intramuscular injections at various time points along with long-term continuous intracerebroventricular infusions using an iPRECIO microinfusion pump (4); this approach effectively cured rabid animals, even in advanced stages of infection.
SYN023, a cocktail of two mAbs (CTB011 and CTB012) against RABV, has been approved in China for PEP of rabies. In this study, we evaluated the clearance efficacy of SYN023 in a murine model challenged with China I strain SC16. Mice were administered SYN023 on day 5 post-inoculation, a late-stage PEP time point at which mice may begin to develop initial symptoms after SC16 injection. Notably, 69% of the treated mice recovered from rabies symptoms and survived until the end of the study with no viral presence in the brain, indicating successful reversal with SYN023.
The RABV strain used in this study, SC16 (GenBank: CSC1016D), was isolated from a dog brain in Sichuan, China, in 2010. SC16 is a representative strain of the China I group, which is the most prevalent and abundant RABV group in China (5). SYN023 (batch number: SYN023DP120210901) is a 1∶1 mixture of two anti-RABV mAbs, CTB011 and CTB012, produced by Synermore Biologics.
The median lethal dose (LD50) of the SC16 RABV was determined by intramuscular injection in BALB/c mice. The virus, diluted 10-fold from 10-1 to 10-5, was injected into the left hind leg muscle of each mouse (10 mice/group). The number of dead mice in each group was recorded daily for 21 days. The LD50 was calculated using the Reed–Muench method (6).
Specific pathogen-free 6-week-old female BALB/c mice were purchased from Vital River Laboratory Animal Technology Co., Ltd. (Beijing), and housed in individual ventilated cages.
For the in vivo murine rabies model, 23 BALB/c mice were intramuscularly injected with 5×LD50 (0.1 mL) of the SC16 virus and randomly divided into a virus control group (n=10) and an SYN023 group (n=13), using a random number generator (https://www.graphpad.com/quickcalcs/randomize1/). Mice in the SYN023 group received daily injections of SYN023 (20 mg/kg) at the virus inoculation site from 5 to 30 days post-infection (dpi). The body weights and symptoms of the test animals were monitored and recorded daily. Animal care staff and administrators were blinded to the allocation groups to ensure that all animals in the experiment were handled, monitored, and treated similarly. Progressive clinical symptoms include ruffled fur, slow movement, hind limb ataxia, apathy, monoplegia, hind limb paralysis, conjunctivitis, and urine staining (4). Brain tissues from the mice that died or were sacrificed at the end of the study were collected for direct fluorescent antibody test (DFA). Blood from surviving mice was collected at termination to measure RABV-neutralizing antibody (RVNA) levels using the rapid fluorescence focus inhibition test (RFFIT).
The RFFIT used a standard challenge virus (CVS-11) and BSR cells. Notably, 50 μL of rabies immunoglobulin and serum (1∶3 dilution) were co-incubated with 50 μL of CVS-11 at 37°C in a 5% CO2 incubator for 1 hour. Subsequently, 50 μL of BSR cell suspension (1×106 cells/m) was added and incubated for 24 hours at 37°C. Cells were fixed, stained with a fluorescent RABV antibody, and observed under a fluorescence microscope. RVNA titers were calculated using the Reed–Muench method (6) and expressed in IU/mL.
To evaluate the therapeutic effect of SYN023 on rabies, the SC16 challenge animal model was first established. Fifty mice were evenly divided into five groups, each comprising 10 mice. These mice were intramuscularly inoculated with serially diluted brain homogenates containing the SC16 strain. All the mice in each group received an injection volume of 0.1 mL. All mice (10/10) in the group inoculated with the highest viral concentration (10-1 dilution) died; all mice (10/10) inoculated with the 10-2 dilution also died. At 10-3 dilution, the mortality rate decreased to 50% (5/10). Remaining groups of mice, inoculated with lower viral concentrations (10-4 and 10-5 dilutions) showed complete survival (0/10 deaths). The DFA test results were positive in the brain tissue of all deceased mice and negative in all surviving mice. The LD50 of SC16 was calculated as 10-3/0.1 mL using the Reed–Muench method.
The SC16 challenge mouse model was used to evaluate the effects of SYN023. Mice infected with the SC16 strain of the RABV were administered either SYN023 (20 mg/kg) or a vehicle control from 5 to 30 dpi. Mice in the control group developed obvious clinical symptoms of rabies from 5 to 7 dpi, and 100% (10/10) of these mice died within 3–6 days of symptom onset. Mice in the SYN023 group demonstrated a markedly improved survival rate of 69% (9/13) by day 30 (P<0.001, log-rank test). The remaining four mice in the SYN023 group died within 7–9 days after symptom onset, which was much longer than that in the control group (Figure 1A and B).
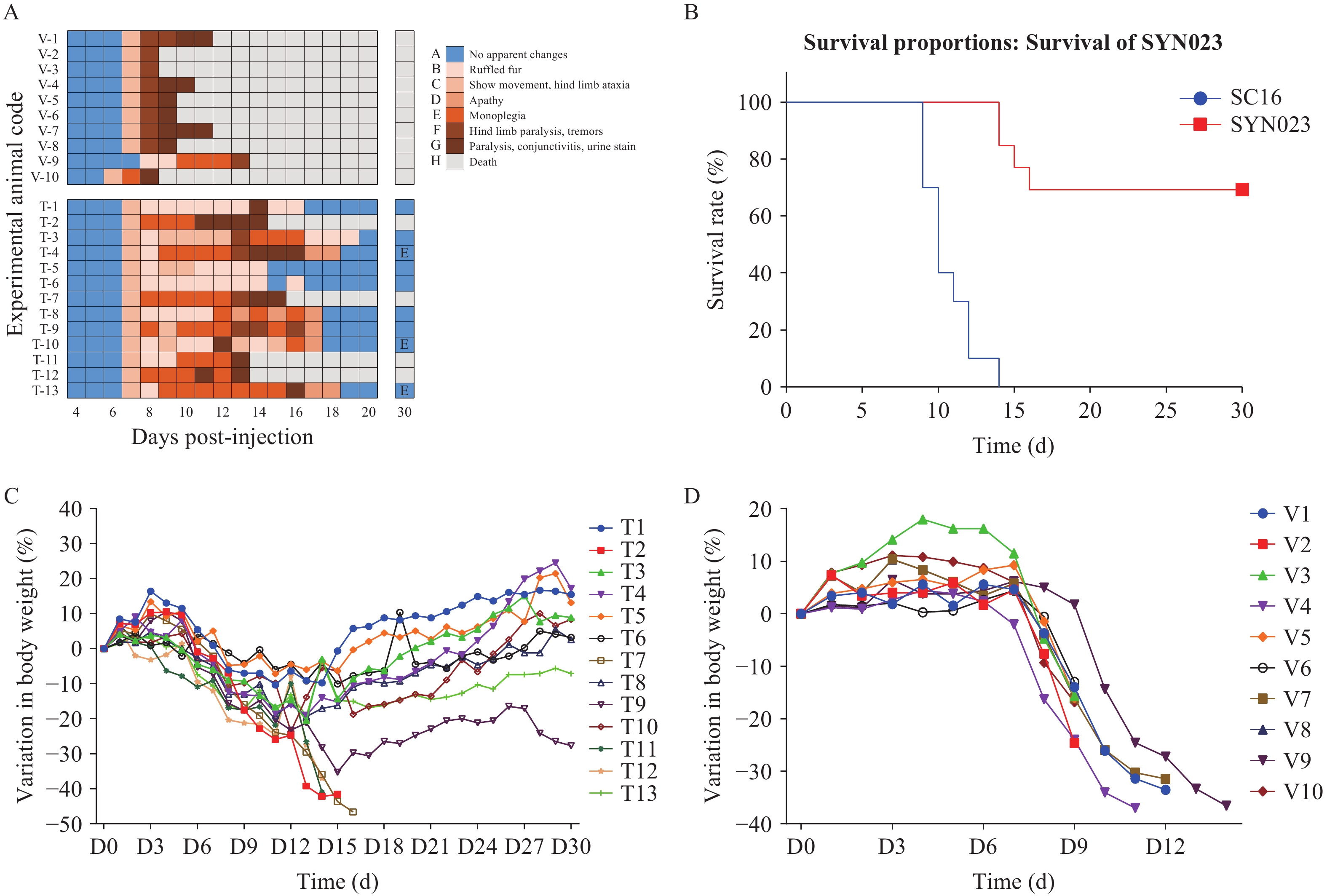 Figure 1.
Figure 1.Therapeutic effect of SYN023 in the SC16 challenge model. (A) Changes in symptoms in the mice in both groups. (B) Survival curves of mice treated with vehicle control or SYN023. (C) Changes in body weight of mice treated with SYN023. (D) Changes in body weight of mice treated with vehicle control.
Note: Survival analysis revealed that SYN023 improved outcomes (69% vs. 0% survival); surviving mice exhibited transient weight loss during the symptomatic phase followed by recovery.Both the groups progressively developed rabies symptoms. Notably, the nine surviving mice in the SYN023 group initially experienced worsening symptoms even after SYN023 administration, ultimately reversing to no apparent symptoms.
All dead mice exhibited significant weight loss before death. Notably, the nine surviving mice in the SYN023 group also lost weight during the symptomatic period, but eventually regained baseline weight after recovery (Figure 1C and 1D).
All mice brain tissues were dissected, and tested using DFA test. Fluorescein isothiocyanate-labeled mAbs against RABV nucleoprotein used for DFA were purchased from Fujirebio, catalog 311520. In the control group, 10 dead mice tested positive for DFA (Figure 2A). In the SYN023 group, four dead mice tested positive for DFA, whereas the nine surviving mice tested negative on day 30 (Figure 2B).
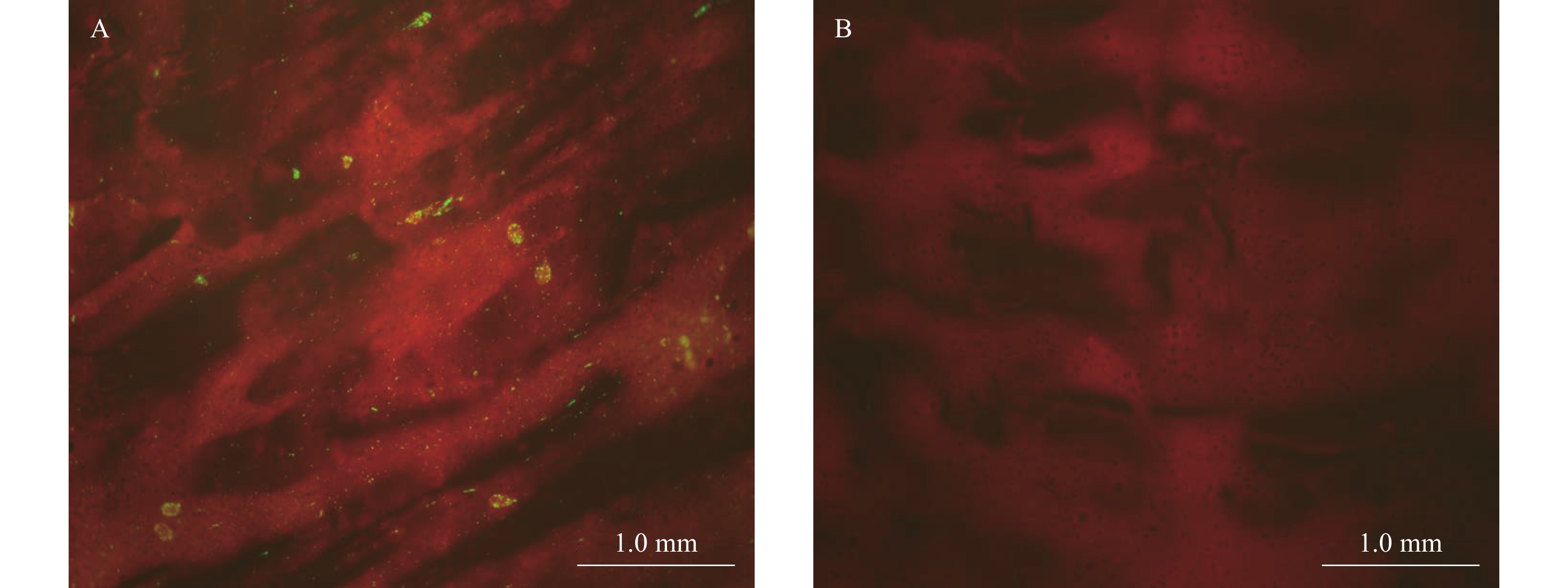 Figure 2.
Figure 2.DFA results from mouse brain tissues. (A) DFA staining of brain imprints from dead mice; (B) DFA staining of brain imprints from surviving mice.
Note: The rabies virus, labeled with an FITC-conjugated monoclonal antibody against its nucleoprotein, exhibits green fluorescence.
Abbreviation: FITC = fluorescein isothiocyanate; DFA= direct fluorescent antibody test.
To further evaluate the effects of SYN023, blood samples were collected from the nine surviving mice in the SYN023 group on day 30 for RVNA detection using RFFIT. As presented in Table 1, the median RVNA titer in the nine surviving mice was 4995.12 IU/mL (range: 2698.89–12535.31 IU/mL), indicating that the sera of the animals maintained high levels of anti-RABV-neutralizing activity following the administration of a high dose of SYN023.
Animal ID RVNA (IU/mL) T1 2,698.89 T3 4,995.12 T4 4,255.64 T5 4,145.78 T6 5,979.24 T8 4,454.98 T9 5,625.22 T10 12,535.31 T13 7,351.75 Abbreviation: RVNA= rabies virus neutralizing antibody. Table 1. Serum RVNA level of the surviving mice in SYN023 group.
-
In 2004, Willoughby from the United States introduced the Milwaukee Protocol, a novel treatment regimen that was considered a potential breakthrough in managing symptomatic rabies cases (7–9). This innovative therapy primarily involves the induction of a medically induced coma and the administration of ketamine and amantadine. However, its efficacy remains inconclusive, and it may cause significant adverse effects; thus, the search for an effective treatment for rabies continues. The combination of RVC20 and RVC58 mAbs has been shown to effectively treat rabies-infected mice via peripheral intramuscular injections in conjunction with long-term continuous intracerebroventricular infusions (4). Neutralization of RABV in the brain and the Fc-mediated immune functions of RVC20 and RVC58 are potential mechanisms of action. In this study, the rescue rate decreased from 100% in the group treated at 6 dpi to 33% in the group treated at 8 dpi, indicating that early administration of mAbs plays a critical role in the reversal process from the onset of rabies symptoms. However, intracerebroventricular administration has a high technical threshold that may limit its clinical application.
SYN023 comprises two mAbs that target two distinct and non-overlapping epitopes, thereby ensuring a broad neutralization spectrum (10). Owing to its high neutralizing titer against RABV, we evaluated its therapeutic effect in a murine model challenged with the RABV strain SC16. SYN023 was administered daily at a dose of 20 mg/kg via intramuscular injection from 5 to 30 dpi with SC16. In the viral control group, all mice succumbed to the disease 3–6 days after symptom onset, whereas in the SYN023 treatment group, four mice (31%) died within 7–9 days following symptom manifestation. Notably, mAb therapy extended the survival intervals in these mice. Additionally, nine mice (69%) in the SYN023-treated group recovered to normal health 15–20 days after symptom onset, with concurrent weight gain as the rabies symptoms subsided. The DFA test confirmed RABV clearance from the brains of surviving mice treated with SYN023. This study demonstrated that even when the optimal PEP window was missed, SYN023 could effectively eliminate the RABV that had entered the central nervous system through high systemic concentrations, thereby producing a significant reversal effect in symptomatic animals. Given the similar survival rate following SYN023 administration via intramuscular injection with that of RVC20 and RVC58 mAbs administration via intramuscular injection and intracerebroventricular infusion, SYN023 could prove a more feasible clinical application. SYN023 has been successfully approved for rabies PEP in China, whereas the clinical development of the RVC20 and RVC58 mAbs was terminated in a phase 2 study. Our findings have important implications for guiding future clinical research, such as combination therapy as an add-on to the Milwaukee Protocol.
This study has several limitations. The cerebrospinal fluid of the nine surviving mice in the SYN023 group was not collected for RVNA detection; thus, the penetration capability of high-dose mAbs across the blood-brain barrier could not be evaluated. The establishment of a dose-response relationship and dosage optimization of SYN023 in a murine rabies model requires further exploration of different dose regimens, administration routes, and durations. Furthermore, in future clinical trials, early administration of SYN023 may depend on the development of more sensitive diagnostic methods for rabies. However, the repeated administration of high-dose mAbs has shown promising efficacy in our murine rabies model. Further basic research will be conducted to explore the mechanism by which mAbs penetrate the blood-brain barrier to treat rabies. In the future, clinical trials should be designed and conducted in patients with rabies using marketed mAbs based on sufficient preclinical studies.
-
Animals were handled in accordance with the Guidelines for the Management and Use of Laboratory Animal Feeding, and the study protocol was reviewed by the Animal Experimental Ethics Committee of the National Institute for Virus Control and Prevention of the Chinese Center for Disease Control and Prevention (license number: sbbdbs20250422055).
HTML
| Citation: |



 Download:
Download:




