2023 Vol. 5, No. 17
A considerable percentage of the population has received both primary and booster vaccinations, which could potentially provide protection against severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) Omicron infections and related symptoms.
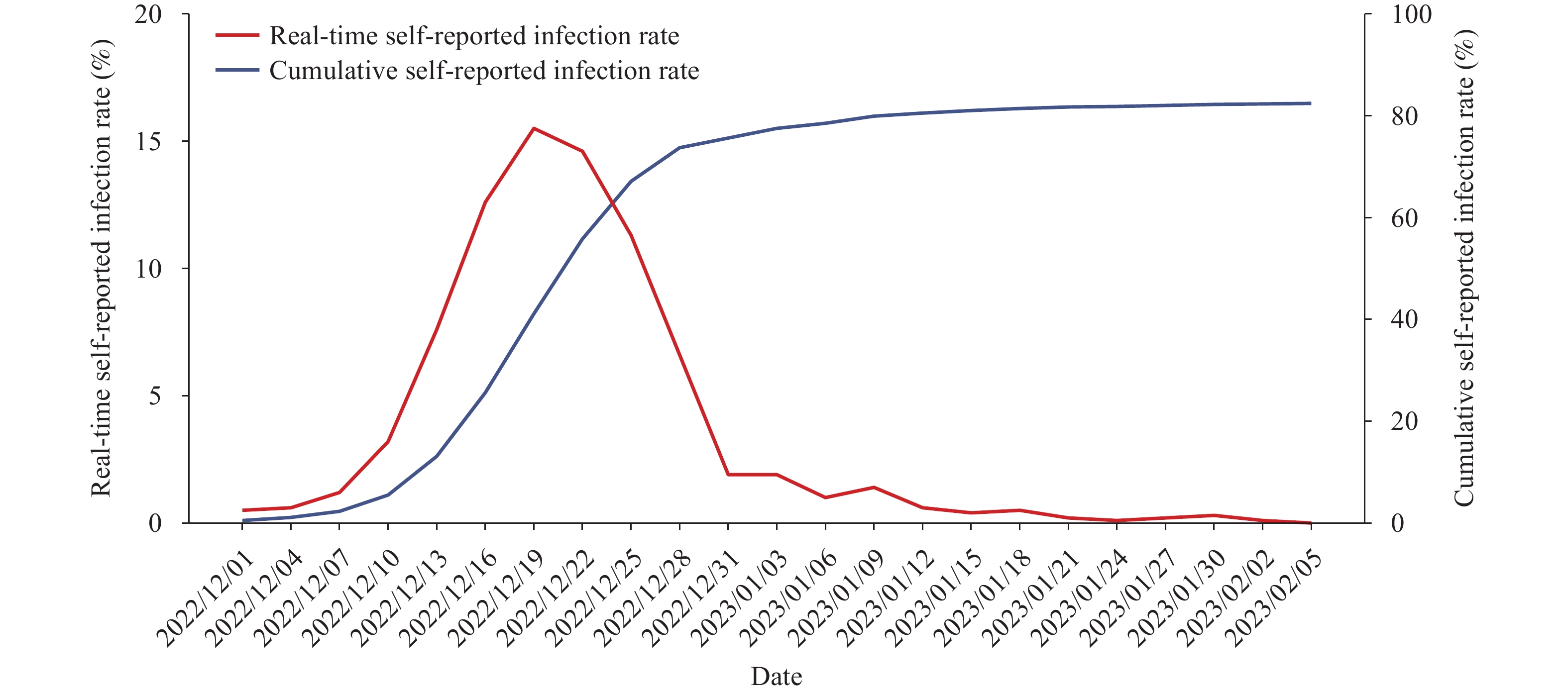
The self-reported infection rate, as determined from an online survey, reached its peak (15.5%) between December 19 and 21, 2022, with an estimated 82.4% of individuals in China being infected as of February 7, 2023. During the epidemic, the effectiveness of booster vaccinations against SARS-CoV-2 Omicron infection was found to be 49.0% within three months of vaccination and 37.9% between 3 and 6 months following vaccination. Furthermore, the vaccine effectiveness of the booster vaccination in relation to symptom prevention varied from 48.7% to 83.2% within three months and from 25.9% to 69.0% between 3 and 6 months post-booster vaccination.
The development and production of efficacious vaccines, together with prompt vaccinations or emergency vaccinations, have the potential to mitigate the epidemic’s impact and safeguard public health.
Vaccine effectiveness (VE) is positively correlated with the number of administered co-purified diphtheria, tetanus, and acellular pertussis vaccine (DTaP) doses. A matched case-control study conducted in Zhongshan City revealed that the co-purified DTaP VE against pertussis-related illnesses in children aged 4–11 months was 42% for one dose, 88% for two doses, and 95% for three doses, respectively.
The results of this study contribute to the current body of research. We found that the VE of co-purified DTaP against pertussis-related illness and hospitalization increased substantially, ranging from 24%–26% after one dose to 86%–87% after four doses.
The results of this study underscore the significance of prompt and comprehensive immunization using co-purified DTaP to decrease the incidence of pertussis. Additionally, these findings offer evidence supporting the modification of China's pertussis vaccination approach.
Limited data exist regarding the coverage of the 13-valent pneumococcal conjugate vaccine (PCV13) in China. A lack of official statistics, coupled with an insufficient body of published literature, hinders the accurate depiction of the current situation.
This study investigated the utilization of PCV13 and estimated its coverage in nine provinces across eastern, central, and western China between 2019 and 2021. Despite an annual increase in PCV13 usage during this period, the overall coverage remained suboptimal.
Consideration should be given to incorporating vaccines into the Expanded Program of Immunization, reducing vaccine prices, and addressing the vaccination coverage gap between eastern and western regions when there is an adequate supply of PCV13, particularly with domestic vaccines.



 Subscribe for E-mail Alerts
Subscribe for E-mail Alerts CCDC Weekly RSS Feed
CCDC Weekly RSS Feed