2025 Vol. 7, No. 3
Type F Clostridium perfringens (C. perfringens) represents a significant pathogen in human gastrointestinal diseases, primarily through its cpe gene encoding C. perfringens enterotoxin (CPE). This investigation examined the prevalence, antimicrobial resistance patterns, and genetic characteristics of Type F C. perfringens within the Chinese population.
The study analyzed 2,068 stool samples collected from 11 provincial hospitals in 2024. Antimicrobial susceptibility testing was conducted following Clinical & Laboratory Standards Institute (CLSI) guidelines, while whole-genome sequencing provided detailed genetic profiles. Evolutionary relationships and clonal transmission patterns were investigated through phylogenetic and genetic environment analyses.
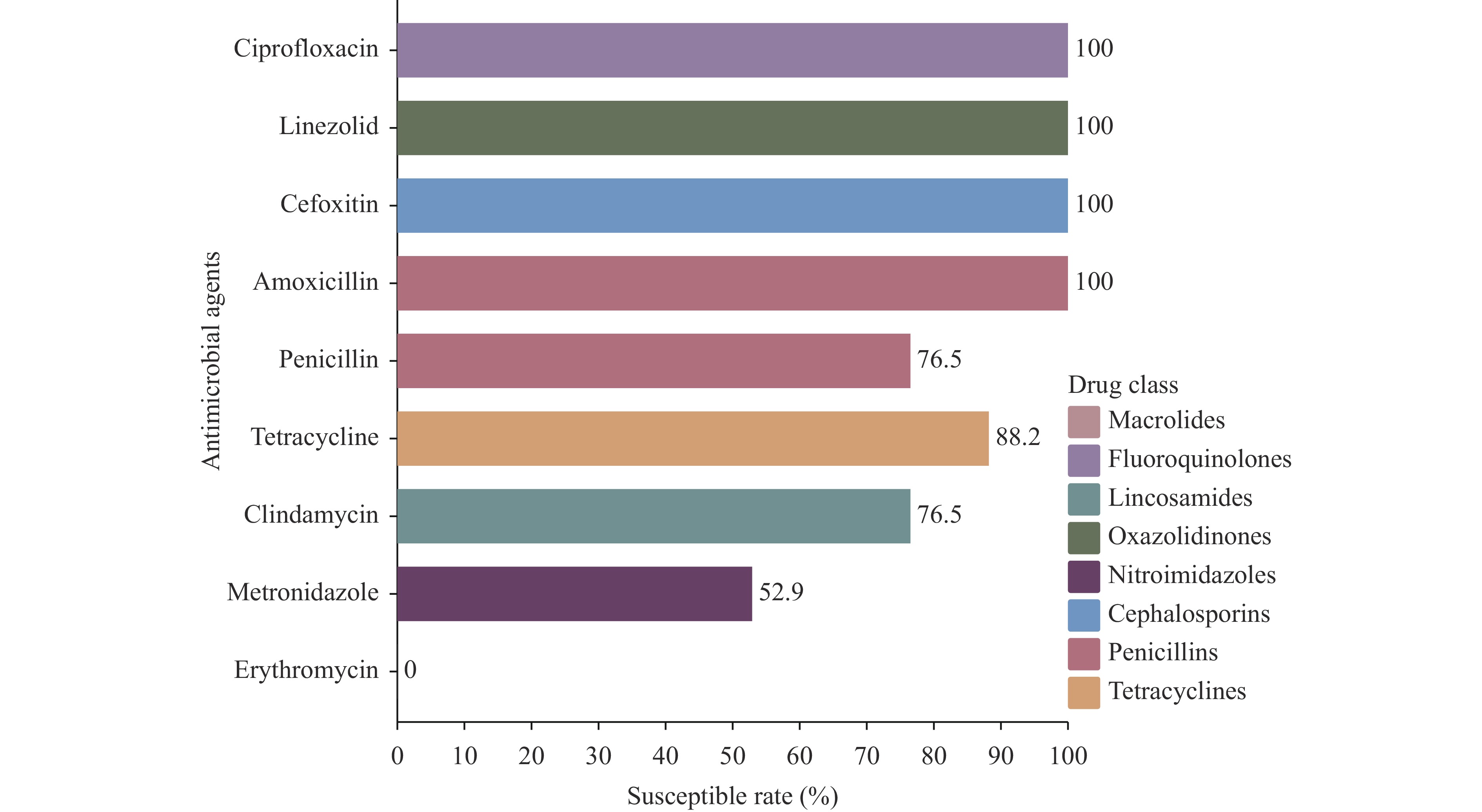
The prevalence of Type F C. perfringens was 2.38%, with isolates predominantly identified in human clinical samples and higher detection rates in gastroenterology departments. Notably, 47.1% of isolates demonstrated high resistance to metronidazole, while all exhibited intermediate resistance to erythromycin. Phylogenetic analysis revealed high similarity among isolates from patients within the same province (single-nucleotide polymorphism (SNPs)<100), and genetic environment analysis indicated potential horizontal gene transfer between animal and human strains.
This investigation predominantly identified Type F C. perfringens in human clinical cases, with sporadic detection in pets and food products. These findings highlight the emergence of Type F C. perfringens outbreaks among diarrheal patients, emphasizing the necessity for targeted interventions as virulence factors increase.
Enterococcus spp., while naturally occurring as commensal bacteria in the gastrointestinal tract of animals and humans, have emerged as significant opportunistic pathogens in healthcare settings.
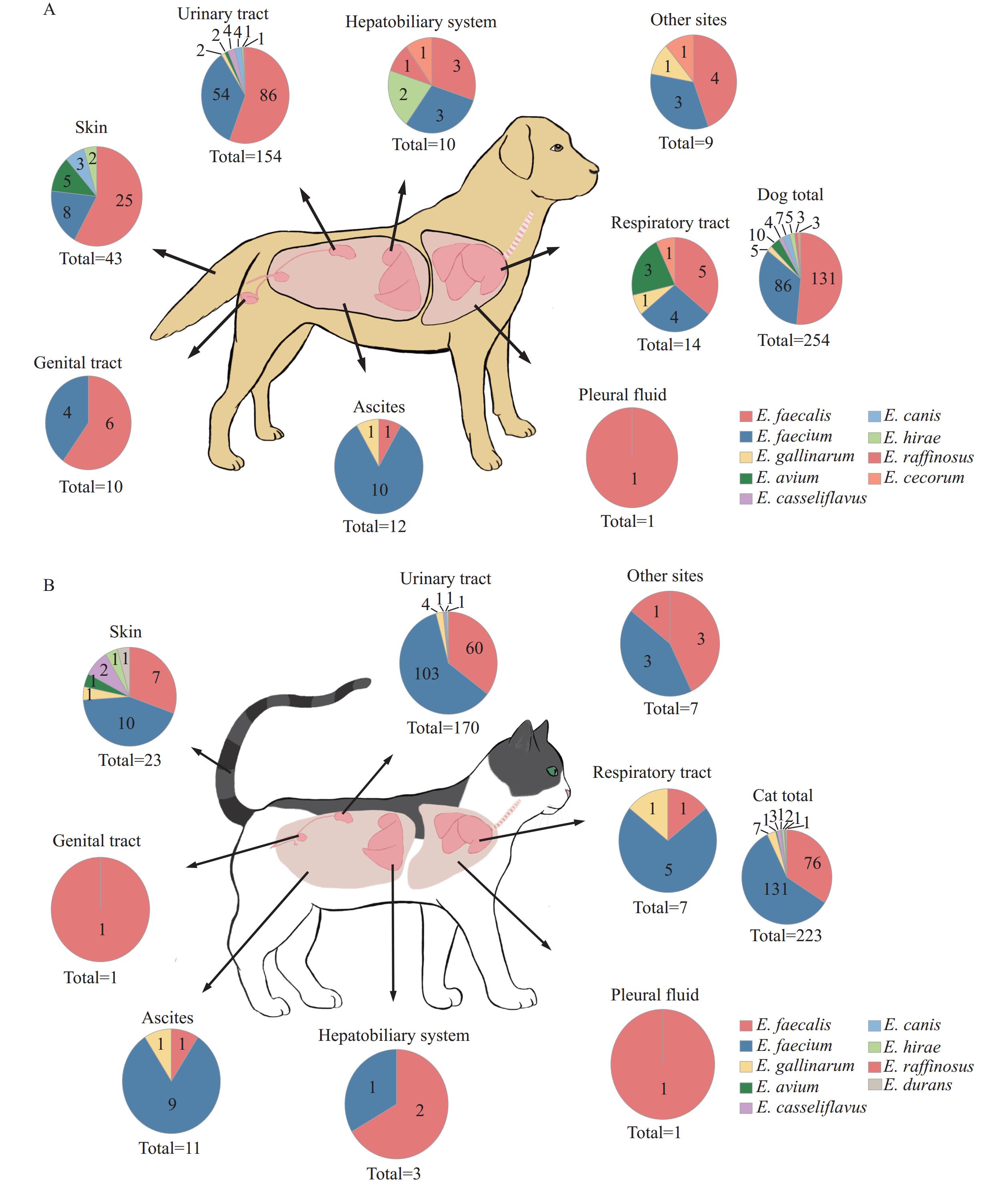
A comprehensive surveillance study revealed enterococci in 14.39% of clinical samples from dogs and cats across China during 2018–2021. Multidrug-resistant enterococcal infections showed significant correlation with urinary tract catheterization and extended hospitalization periods. Notably, pet-derived Enterococcus faecalis isolates demonstrated high genetic similarity with strains isolated from humans, farm animals, and environmental sources.
These findings underscore the critical need for enhanced surveillance of enterococcal infections and implementation of stringent aseptic protocols in veterinary clinical settings. Particular attention should be directed toward linezolid-resistant Enterococcus faecalis infections due to their demonstrated potential for transmission between pets and humans.
Intestinal infections affect approximately 450 million people globally, predominantly impacting children and immunocompromised individuals in low- and middle-income countries (LMICs) due to inadequate water, sanitation, and hygiene (WASH) conditions, poverty, malnutrition, and low literacy. In Kenya, the prevalence of intestinal infections is elevated by warm tropical climates and socioeconomic factors. This scoping review evaluates the national prevalence, risk factors, and contamination sources of intestinal protozoa in Kenya, using a One Health approach to synthesize existing data from various human, animal, and environmental studies. A comprehensive literature search identified 292 studies, of which 67 met the inclusion criteria, covering the period from 1966 to 2024. The review found that most studies utilized stool microscopy, a method with limited sensitivity, and largely focused on vulnerable human populations, with minimal investigation into environmental reservoirs. Key protozoa identified included Entamoeba histolytica, Cryptosporidium, and Giardia, with transmission driven by poor WASH conditions, environmental factors, and close human-animal interactions. The findings highlight significant gaps in environmental surveillance and suggest the need for a robust, integrated One Health approach to better understand and control protozoan infections in Kenya.
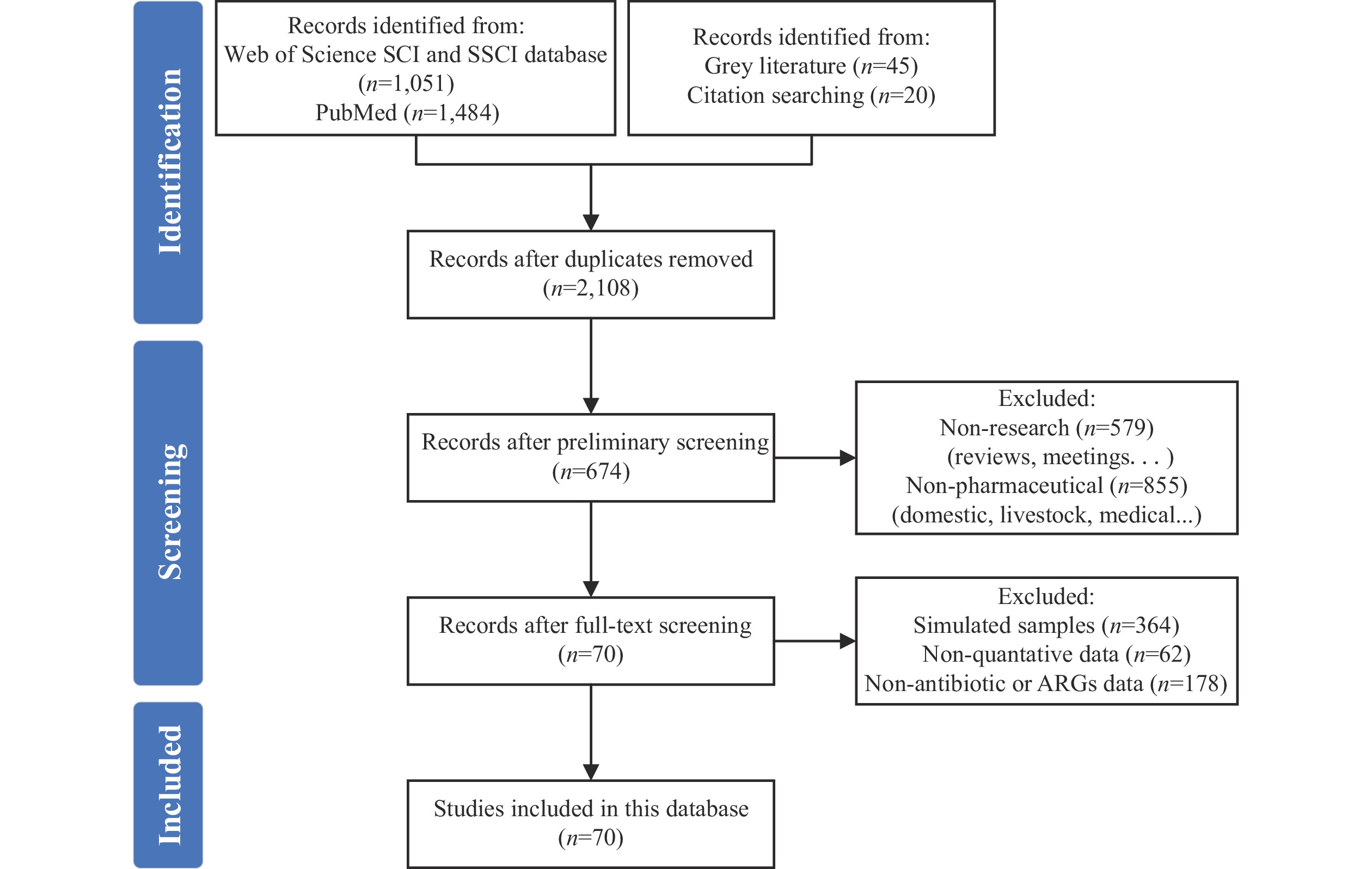
Residual antimicrobial agents in wastewater and solid waste from antimicrobial manufacturing facilities can potentially contaminate environments. The World Health Organization has established technical guidelines for managing antimicrobial resistance (AMR) in pharmaceutical wastewater and solid waste. However, the scarcity of publicly available data on antimicrobial manufacturing processes impedes the development of effective mitigation strategies. To address this knowledge gap, we developed a comprehensive database documenting antibiotics and antibiotic resistance genes (ARGs) in actual wastewater and solid waste samples, primarily fermentation residues. Through systematic review methodology, we compiled data from extensive searches of English-language article databases, including Web of Science and PubMed. The database contains data from 270 distinct samples collected across 45 fermentation residue treatment systems and 46 wastewater treatment systems, derived from 70 published English-language articles spanning 2008 to 2024. In operational pharmaceutical facilities, antibiotic concentrations ranged from 82 to 1,663 mg/L in raw wastewater and from 1,000 to 10,182 mg/kg dry matter (DM) in antibiotic fermentation residues. Various treatment technologies demonstrated significant reductions in both antibiotic concentrations and ARG levels within wastes. This database provides the first global perspective on antibiotic and ARG contamination from antibiotic production processes, supporting AMR management initiatives. It establishes a dynamic, continuously updated platform accessible to researchers and industry stakeholders via the link: https://dash.drwater.net/antiboard/.
The establishment of a high-throughput quantification approach for waterborne pathogenic protozoa and helminths is crucial for rapid screening and health risk assessment.
We developed a high-throughput quantitative polymerase chain reaction (HT-qPCR) assay targeting 19 waterborne protozoa and 3 waterborne helminths and validated its sensitivity, specificity, and repeatability. The assay was then applied to test various environmental media samples.
The HT-qPCR assay's limit of detection (LOD) was 5×102 copies/μL DNA, and its specificity was confirmed using Giardia and Cryptosporidium standards. Repeatability, assessed through intra- and inter-group experiments, yielded a coefficient of variation (CV) of 1.0%–4.6% and 1.2%–6.4% at concentrations of 1×105 and 1×104 copies/μL, respectively. The R2 values of the 22 standard curves ranged from 0.983 to 0.998, with amplification efficiencies between 80% and 107%. In drinking water sources, sludge from municipal wastewater treatment plants (MWTPs), and livestock manure samples, 17 of 22 targets were detected, with Acanthamoeba genus (50.0%), Acanthamoeba castellanii (11.8%), and Enterocytozoon bieneusi (11.8%) showing high prevalence. Cryptosporidium spp., Enterocytozoon bieneusi, and Cyclospora cayetanensis were simultaneously found in all three sample types.
This study presents a useful tool for the rapid detection of waterborne protozoa and helminths in complex environmental microbiomes, providing scientific data for monitoring cross-media transmission and controlling microbial risk from a One Health perspective.
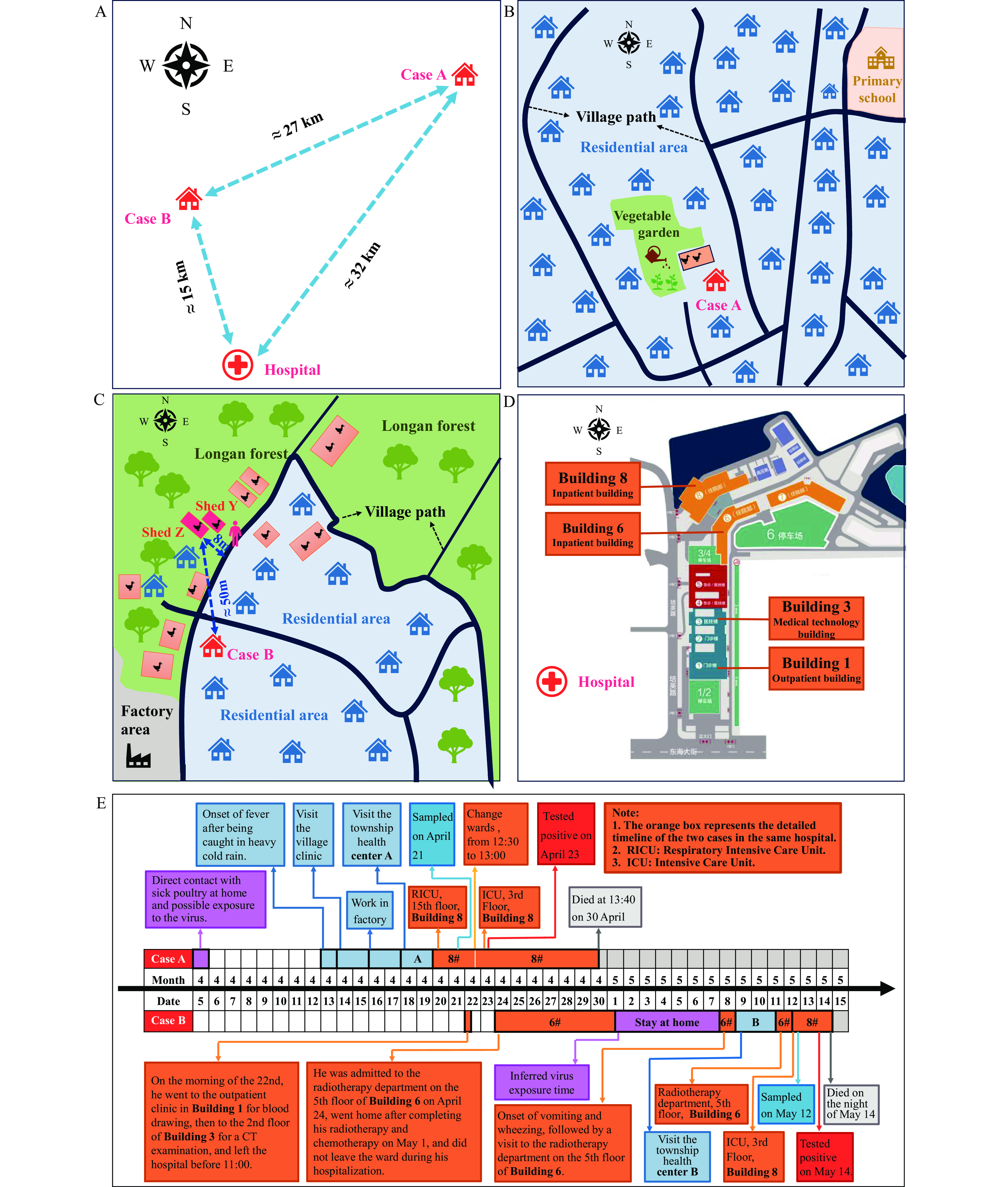
Global human cases of zoonotic influenza A(H5N6) have increased significantly in recent years, primarily due to widespread circulation of clade 2.3.4.4b virus since 2020. Concurrent with this trend, sporadic human infections with clade 2.3.4.4h H5N6 avian influenza virus continue to occur. The high mortality rate associated with H5N6 virus infections has emerged as a critical public health concern.
Through comprehensive field epidemiological investigations and laboratory analyses, we identified the infection sources for these cases and conclusively ruled out human-to-human transmission. Genetic analyses revealed that while the virus maintains its avian host tropism, it has acquired mutations that may enhance human receptor binding affinity, viral replication capacity, pathogenicity, and neuraminidase inhibitor resistance.
The ongoing viral mutations increase the potential for H5 subtype avian influenza viruses to overcome species barriers and cause human epidemics. Enhanced surveillance strategies incorporating advanced technologies, such as metagenomic sequencing, are essential for early risk detection and management. Special attention should be directed toward cancer patients and immunocompromised individuals, who demonstrate increased susceptibility to avian influenza virus infections and require targeted prevention and control measures.



 Subscribe for E-mail Alerts
Subscribe for E-mail Alerts CCDC Weekly RSS Feed
CCDC Weekly RSS Feed