-
Antimicrobial resistance (AMR), driven by high concentrations of active pharmaceutical ingredients (API), poses a significant threat to disease treatment effectiveness (1–2). Pharmaceutical manufacturing processes release substantial quantities of antibiotics into the environment through wastewater and solid waste, with fermentation residue representing a major solid waste component during antibiotic production. The complex matrix of contaminants and microbiota in these waste streams presents significant management challenges. The World Health Organization (WHO) and the United Nations Environment Programme (UNEP) jointly released the Guidance on wastewater and solid waste management for manufacturing of antibiotics (3) in September 2024, highlighting antibiotic production’s crucial role in global AMR surveillance and control.
Antibiotic manufacturing facilities require dedicated collection and treatment infrastructure for both wastewater and solid waste streams, providing opportunities to control the environmental release of antibiotics and antibiotic resistance genes (ARGs) (4–6). However, comprehensive data and scientific understanding of AMR pollution characteristics within the pharmaceutical industry remain limited. To address this knowledge gap, we developed a systematic review-based database documenting antibiotics and ARGs in pharmaceutical wastewater and solid waste streams. Fermentation residues constitute the primary solid waste generated during antibiotic production processes. The database development incorporated diverse scientific literature, including English-language review articles and research papers on treatment technologies and applications for pharmaceutical wastewater and solid waste (5,7), as well as relevant grey literature from local, national, and regional sources (8–10). China has emerged as a leader in this field, demonstrating substantial research achievements and extensive international collaboration. This database serves as an essential resource for understanding the current status of antibiotics and ARGs in pharmaceutical manufacturing while supporting enhanced management and control strategies.
-
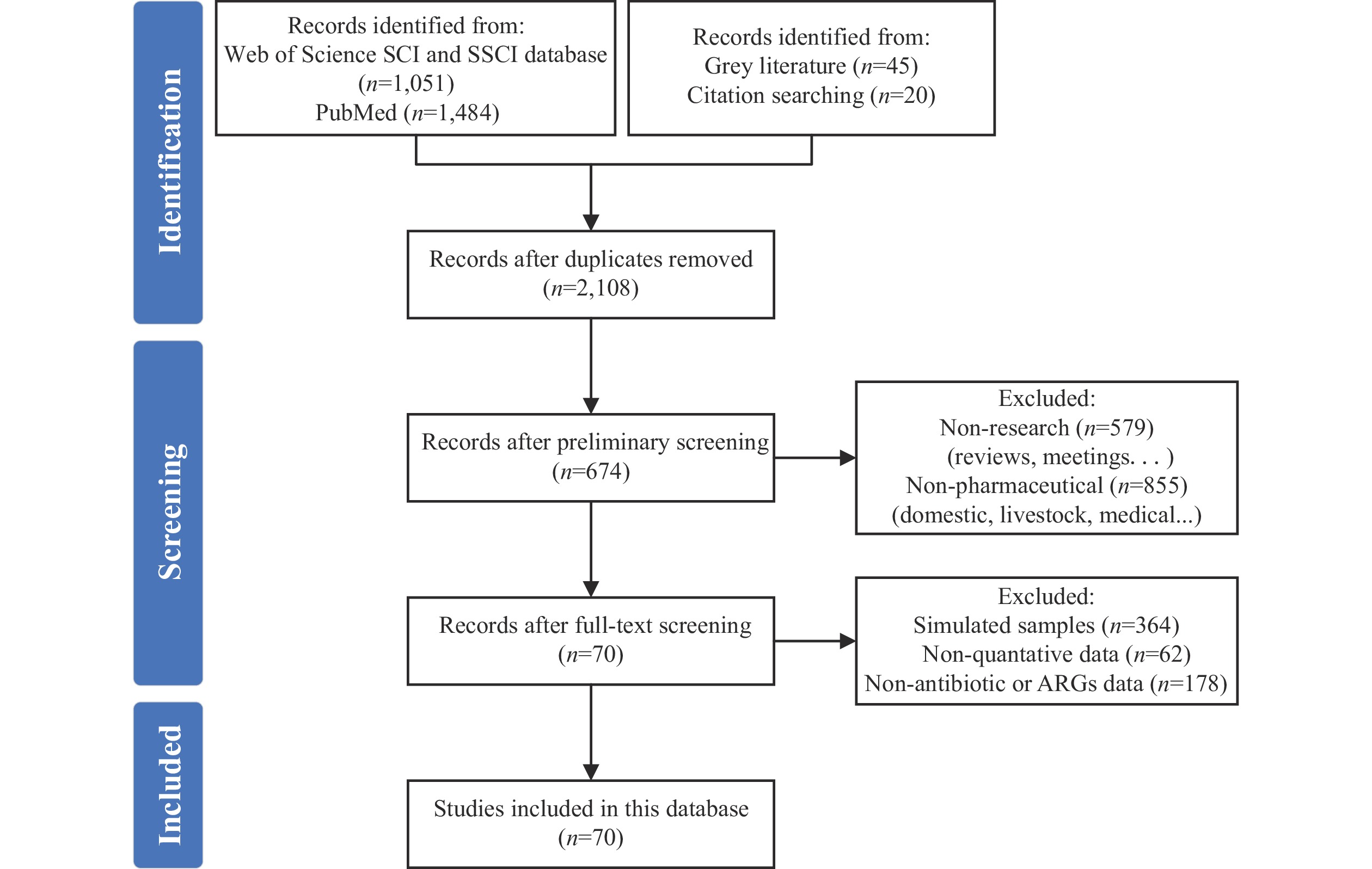
Following the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines (11), we conducted a comprehensive literature search using the Science Citation Index (SCI) and Social Sciences Citation Index (SSCI) databases in Web of Science (WOS) and PubMed. The search covered articles published from January 2000 to December 2023. In WOS, we employed the following search strategy: “TS = {[(pharmaceutic* OR production OR fermentation) NEAR (waste* OR residue OR mycelia)] NEAR antibiotic*}”, with a comparable approach used in PubMed. We supplemented these database searches with relevant grey literature (Figure 1). Using CiteSpace (12), we analyzed and visualized collaboration networks among countries, institutions, and researchers based on the 1,000 most relevant papers.
-
Analysis of publication trends revealed 2,108 unique publications following duplicate removal, demonstrating a marked increase during the 2000–2023 period (Figure 2A). Prior to 2015, research on antibiotic production wastes received limited attention, with minimal publications. From 2016 onward, publication rates exhibited a significant upward trajectory. Publication volumes in 2022 and 2023 exceeded 320 annually, representing more than a fourfold increase compared to 2015’s 79 publications. This trend indicates the growing recognition of pharmaceutical waste management as a critical research focus.
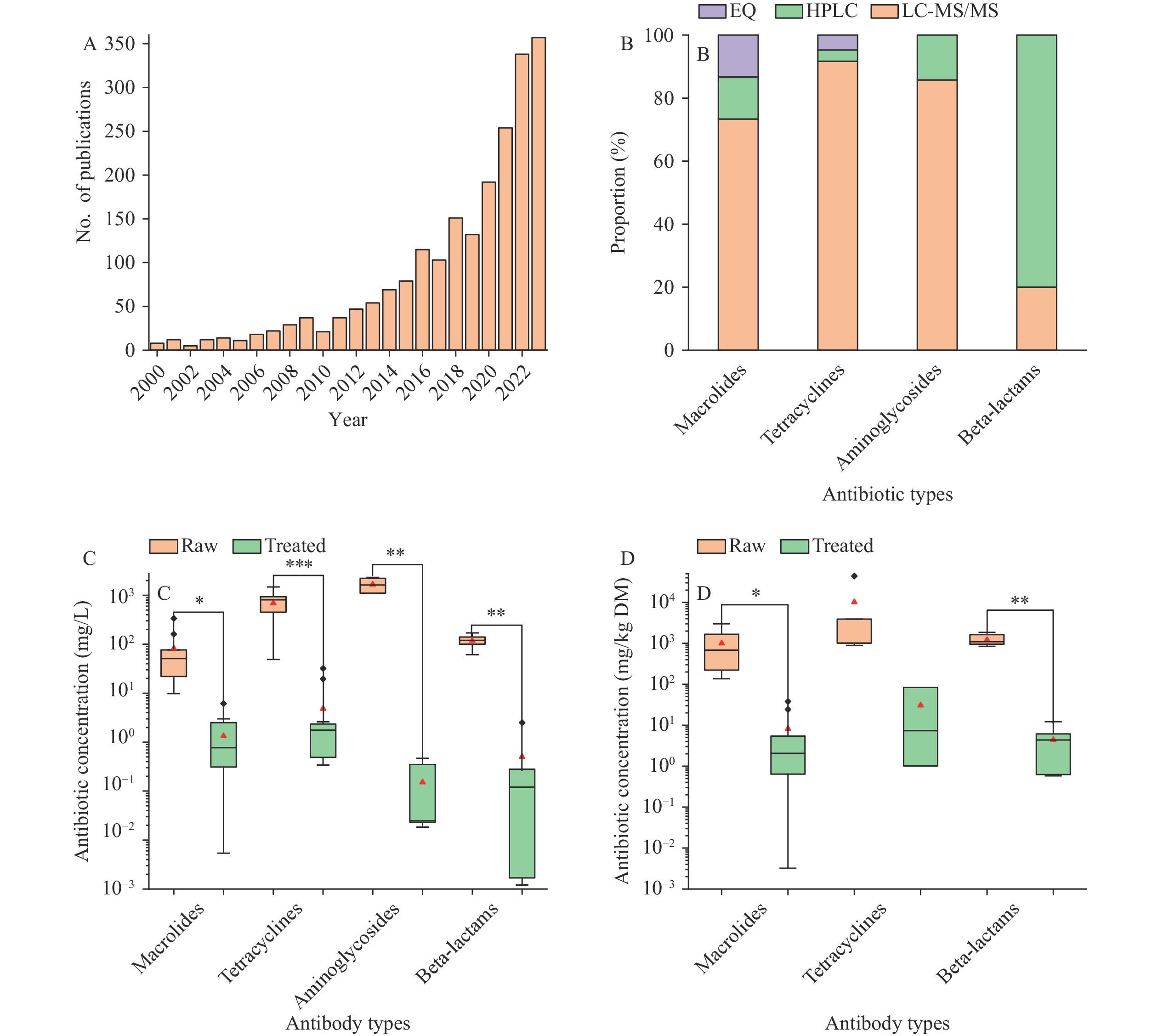 Figure 2.
Figure 2.Analysis of publication trends and antibiotic measurements. (A) Annual publication numbers; (B) Distribution of antibiotic detection methods; (C) Antibiotic concentrations in pharmaceutical wastewater; (D) Antibiotic concentrations in fermentation residues.
Note: Red triangles (C, D) indicate mean values; black squares represent outliers. Statistical significance of pre- versus post-treatment concentration differences was determined using Paired-Sample t-Tests, with asterisks denoting significance levels.
Abbreviation: EQ=equivalent quantity; HPLC=high-performance liquid chromatography; LC-MS/MS=Liquid Chromatography-Mass Spectrometry/Mass Spectrometry.
China emerged as a pioneer in addressing pharmaceutical wastewater and residue management, with Chinese researchers contributing 371 publications, substantially surpassing other nations. China’s pivotal role in international collaboration is evidenced by its high betweenness centrality (0.54), calculated from collaboration frequency. India ranked second with 87 publications (centrality 0.18), followed by the United States with 63 publications (centrality 0.35). Notably, while Iran produced 56 publications, its low centrality (0.13) indicates limited international collaboration. Conversely, Spain demonstrated strong international engagement with high centrality (0.32) despite fewer publications (Table 1).
Country/Institution/Author Publications Centrality Year of first publication Country China 371 0.54 2007 India 87 0.18 2002 Spain 33 0.32 2006 France 30 0.08 2010 Brazil 29 0.07 2009 America 63 0.35 2002 Iran 56 0.13 2014 England 23 0.22 2001 Institution Chinese Academy of Sciences 78 0.02 2008 Research Center for Eco-Environmental Sciences (RCEES) 41 0.08 2008 Tsinghua University 39 0.05 2013 University of Chinese Academy of Sciences 31 0.03 2017 Istanbul Technical University 16 0.08 2004 Indian Institute of Technology System (IIT System) 10 0.01 2016 Author Yang, Min 24 0.13 2008 Zhang, Yu 20 0.08 2008 Table 1. International collaboration analysis across countries, institutions, and researchers.
Within China’s research landscape, the Chinese Academy of Sciences (CAS) and Tsinghua University (THU) distinguished themselves through substantial publication output and high centrality metrics. The University of Chinese Academy of Sciences (UCAS) and the Research Center for Eco-Environmental Sciences (RCEES), both CAS affiliates, achieved significant research outcomes. The research group led by Min Yang and Yu Zhang has gained particular prominence, earning substantial citations for their sustained investigations into pharmaceutical wastewater and fermentation residue. In India, the Indian Institute of Technology System (IIT System) and in Turkey, Istanbul Technical University, emerged as leading institutions. While China and India dominate bulk antibiotic production, the challenges of antibiotic pollution and AMR risks have attracted significant attention from international organizations including UNEP and WHO, as well as industry groups like the Antimicrobial Resistance Industry Alliance, resulting in comprehensive guidelines addressing pharmaceutical supply chain pollution (8,13). The IIT system in India has conducted extensive research on local pharmaceutical wastewater treatment systems, emphasizing the crucial role of regulatory standards in AMR control (14).
-
Following PRISMA guidelines, 70 papers reporting actual samples from 2008-2024 were incorporated into the database (Figure 1). The database comprises 16 comprehensive columns documenting: antibiotic and sample types, geographical location, studied systems, treatment methodologies, experimental scale, detection methods, antibiotic concentrations, ARG testing methods and results, publication year, source ID, and reference source (Figure 3). Each entry’s source ID links directly to detailed publication information, facilitating efficient data retrieval.
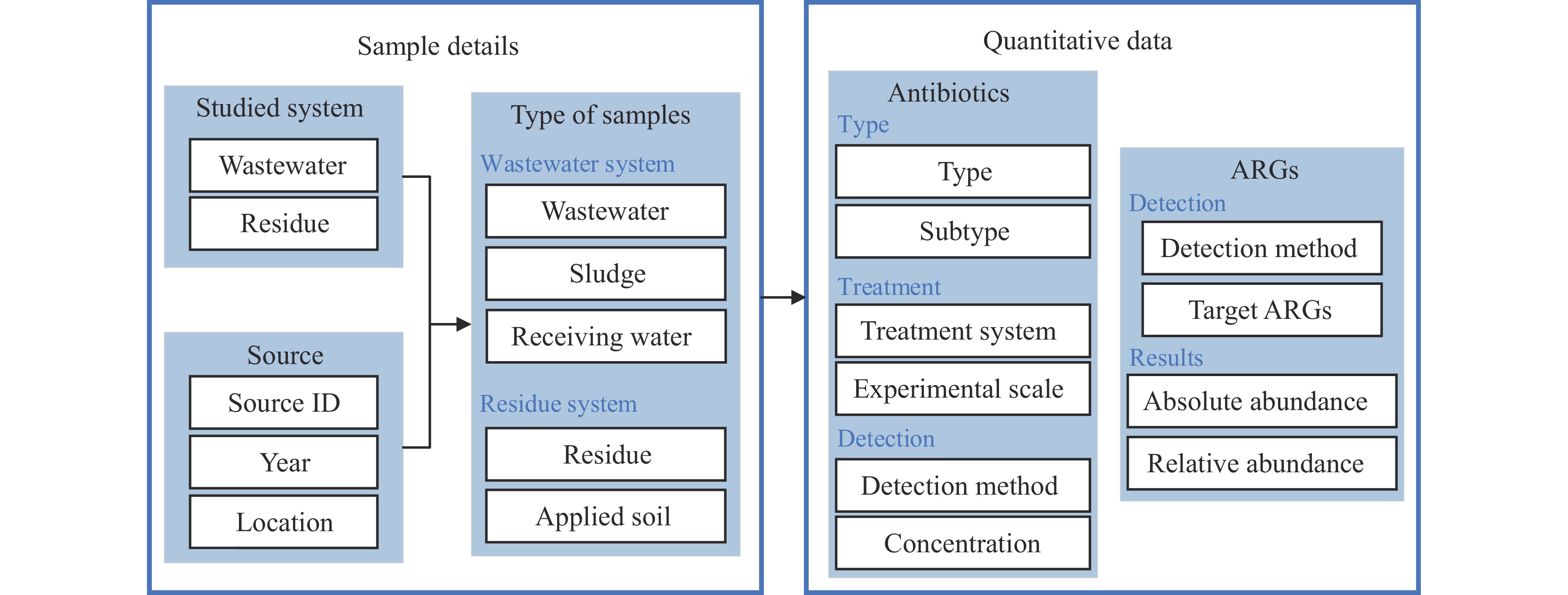 Figure 3.
Figure 3.Framework of the original and quantitative samples data.
Abbreviation: ARGs=antibiotic resistance genes.The database encompasses 270 samples across five major categories: wastewater, sludge, and receiving water from wastewater treatment systems; and fermentation residue and applied soil from fermentation residue research systems. It contains 256 antibiotic records and 100 ARG records. The antibiotic records are distributed as follows: 96 for residues, 1 for applied soil, 126 for wastewater, 26 for sludge, and 7 for receiving water. ARG documentation includes 39 records for residues, 3 for applied soil, 31 for wastewater, 27 for sludge, with no records for receiving water. The most extensive antibiotic concentration datasets were collected in 2023 (n=50), 2015 (n=30), and 2013 (n=21). Similarly, ARG data peaked in 2023 (n=28), followed by 2019 (n=17) and 2013 (n=16). Sampling locations primarily span 14 provincial-level administrative divisions (PLADs) in China, with a concentration in the northern region due to the prevalence of antibiotic production facilities. Additional reports originated from Poland and Croatia.
The geographical distribution of pharmaceutical facilities shows a significant dispersion, with notable clustering in northern China. Manufacturing facilities in Hebei Province and Shandong Province demonstrate diverse production capabilities, manufacturing antibiotics across five major categories. Macrolide and aminoglycoside production facilities show the broadest geographical distribution, operating across six PLADs or municipalities. Tetracycline and β-lactam production facilities are distributed across five PLADs or municipalities each.
-
The primary quantitative methods for antibiotic detection comprise liquid chromatography-tandem mass spectrometry (LC-MS/MS) and high-performance liquid chromatography (HPLC) (Figure 2B). The selection of detection methodology is determined by the specific antibiotic type and molecular structure. Ultra-performance liquid chromatography-tandem mass spectrometry (UPLC-MS/MS) is preferentially employed for macrolides, tetracyclines, and aminoglycosides due to its superior sensitivity. Conversely, HPLC enables precise separation and quantitative analysis of β-lactam antibiotics in complex matrices. Additionally, Zhang et al. (15) introduced an innovative potency assay method to evaluate residual antibacterial effects, expressed as antibiotic equivalent quantity (EQ). Compared to conventional chemical methods, the EQ approach comprehensively accounts for the antibacterial activities of both parent compounds and their transformation products.
Antibiotic concentrations in production wastewaters were substantially elevated, with mean values ranging from 82 to 1,663 mg/L, markedly higher than the μg/L levels typically observed in municipal and domestic wastewaters (16–18). Tetracycline and aminoglycoside antibiotics exhibited particularly high concentrations (Figure 2C). Post-treatment analysis revealed significant reduction in residual antibiotics, with mean effluent concentrations below 5.0 mg/L across various antibiotic classes. However, these levels remained substantially higher than the 12.5 μg/L typically found in domestic wastewater treatment plant effluent (19).
Among fermentation residues, macrolides, tetracyclines, and beta-lactam antibiotics have been most extensively investigated. Raw sample concentrations averaged between 1,000 to 10,182 mg/kg dry matter (DM), substantially higher than the 4.0 mg/kg observed in municipal sewage sludge (20). Hydrothermal treatment and other remediation approaches achieved removal rates exceeding 90% (Figure 2D). Given the potential ecological risks and antimicrobial resistance concerns, fermentation residue has been classified as hazardous waste requiring efficient remediation treatment.
Researchers primarily employed quantitative PCR (qPCR) and metagenomic sequencing for ARG detection. Both production wastewater and fermentation residues exhibited significant ARG enrichment, predominantly showing resistance to the antibiotics being manufactured (Table 2). While biological treatment systems effectively removed antibiotics and certain ARGs from wastewater (5,21), treatment system sludge showed ARG enrichment, with abundance levels surpassing those found in fermentation residue from the same facility (22). ARG abundance in fermentation residues consistently reached approximately 5 log copies/mg.
Antibiotic Sample Methods Target ARGs Abundance Ref. SPM Sludge qPCR 4 erm, ereA, mphB, mefA, msrD 1.4×107 copies/mg; 4.3×10−1 copies/16S rRNA gene (22) Residue qPCR 4 erm, msrD 1.6×105 copies/mg; 1.6×10−3 copies/16S rRNA gene Residue qPCR ereA, 2 erm, mefA 1.64×107 copies/mL,decrease 83.6% (38) Compost qPCR 4 erm, mefA, mphA Lowest in mature phase (39) ERY Effluent qPCR 5 erm 8 log copies/mL (40) Residue qPCR 2 erm, ereA, mpfB, mefA 1.45–5.68 log copies/mL, decreased by
96%–99% after treatment.(41) Residue qPCR 2 erm, ereB 7.42×10−9−6.82×10−4 copies/16S rRNA gene,
decreased 99% after treatment.(42) Effluent HT-PCR 3 erm, maA/mel, floR, sul2, tetM, mefA 1.8–5 log copies/mL (43) Influent qPCR 4 erm, 1 ere, 2 mph, 1 mef 4.3×108 copies/mL (5) Effluent qPCR 4 erm, 1 ere, 2 mph, 1 mef 2.1×107 copies/mL OTC Influent qPCR 9 tet 7.0×109 copies/mL; 3.2×100 copies/16S rRNA gene (21) Effluent qPCR 9 tet 1.8×108 copies/mL; 1.4×100 copies/16S rRNA gene Residue qPCR 9 tet 1.4×105 copies/mg; 2.4×10−2 copies/16S rRNA gene Sludge qPCR 5 tet 9-13 log copies/g dry matter (44) Residue qPCR 3 tet 6.5×105 copies/mg in raw residue, decreased to
1.28×104 copies/mg after treatment.(45) Compost Metagenomics Multi-drugs After composing, multiple ARGs increased by 57.38 %. (46) Sludge Metagenomics sul1, aph(6)-I, tetA Total: 2.41-6.55 copies/16S rRNA gene
Tetracycline:0.82±0.21 copies/16S rRNA gene(47) TC Production wastewater qPCR 2 sul, 4 tet, 2 bla, ermB, qnrD Average (4.80 ± 12.84) × 105 copies/mL each. (48) Effluent qPCR 2 sul, 3 tet, 2 bla, qnrD 0.4-5.1×107 copies/mL each. PG Residue qPCR blaTEM 4.17±0.19 log copies/mg (49) Compost qPCR blaTEM 8.98±0.27 log copies/mg Abbreviation: SPM=spiramycin; ERY=erythromycin; OTC=oxytetracycline; TC=tetracycline; PG=penicillin; qPCR=quantitative polymerase chain reaction; ARGs=antibiotic resistance genes; Ref.=reference. Table 2. Detection of antibiotic resistance genes in pharmaceutical-related matrices.
Notably, treatment experiments remain predominantly confined to laboratory scale. Among research groups, the RCEES, CAS team has conducted the most extensive field studies investigating antibiotic and ARG occurrence and removal, with the explicit goal of scaling findings to pilot and full-scale applications (4,23–26).
-
Biological treatment processes, particularly the combination of anaerobic digestion and activated sludge treatment, represent the predominant approach for managing antibiotic-containing pharmaceutical wastewater (5,21,27). However, high concentrations of antibiotic residues in wastewater have been demonstrated to promote ARG development and lead to treatment system failure (4,28).
To address this challenge, pretreatment of production wastewater to remove antibiotics prior to biological treatment has emerged as the optimal strategy for controlling ARG development (8,29-30). Enhanced hydrolysis has established itself as the leading pretreatment method in full-scale pharmaceutical wastewater treatment systems, demonstrating removal efficiencies exceeding 99% for oxytetracycline (4,31-32). For fermentation residues, hydrothermal treatment has shown exceptional effectiveness in eliminating both antibiotics and ARGs, achieving approximately 90% removal efficiency (33-34).
-
To ensure data accuracy and validity across diverse reporting formats, we implemented a comprehensive validation protocol. For ARG data, we maintained distinct recording formats: specific target genes for qPCR analyses and comprehensive gene profiles for metagenomic studies. Sample-specific units were standardized according to matrix type (mass-based for solids and volume-based for liquids). We employed a dual-verification system where one researcher entered the records while a second researcher independently validated the dataset to prevent errors and eliminate duplicate entries.
-
This comprehensive database encompasses 270 records documenting antibiotics and ARGs in pharmaceutical wastewater and solid waste, developed through systematic literature review. The database is accessible through an online platform https://dash.drwater.net/antiboard/, providing researchers with validated scientific data for analysis and reference. The Environmental Microbiology Technology Research Group of RCEES, CAS maintains continuous data collection and performs periodic updates to the website. Future development of the database aims to enhance pharmaceutical industry management through collaborative support from pharmaceutical industry associations.
This study provides a comprehensive overview of antibiotic production industry waste research and establishes a database tracking antibiotics and ARGs in actual industrial samples. While numerous treatment technologies demonstrate promising laboratory-scale removal efficiencies for both antibiotics and ARGs, validation through full-scale implementation remains crucial. Given that India and China dominate global antimicrobial manufacturing, enhancing pollution control measures in these nations offers the greatest potential for reducing worldwide AMR risks from pharmaceutical production. The implementation of enhanced hydrolysis pre-treatment to reduce antibiotic concentrations in manufacturing wastewater has proven particularly effective in controlling AMR development during biological treatment. This approach has been successfully scaled to full-scale wastewater treatment facilities in China, positioning Chinese AMR prevention technologies at the forefront of global pharmaceutical industry practices. As the field continues to evolve, this database serves as a vital resource for researchers and industry stakeholders addressing these challenges.
In 2008, while China’s Ministry of Environmental Protection established discharge limits for key pollutants in pharmaceutical wastewater, the absence of specific standards for residual antibiotics highlighted the need for further research on resistance thresholds (35). The Ministry of Ecology and Environment’s 2023 technical guidelines for wastewater treatment represent progress, though many emerging technologies remain in experimental phases (10). Notably, antibiotic fermentation residues have maintained their classification as hazardous waste since their initial inclusion in the Directory of National Hazardous Wastes in 2008.
The current database iteration, while ensuring accuracy through manual literature curation, faces challenges in keeping pace with the rapidly expanding volume of publications on pharmaceutical industry antibiotic pollution and resistance. Integration of artificial intelligence (AI), particularly Large Language Models (LLMs) and Retrieval-Augmented Generation (RAG) systems, offers potential solutions for enhancing data retrieval, automating updates, and transforming the database into a dynamic, self-updating knowledge system (36-37). Furthermore, future expansions will incorporate data from Chinese theses, dissertations, and grey literature, including relevant local, national, and regional documents, extending beyond the current English-language research paper focus.
HTML
Data Records
Detection Methods and Concentration of Antibiotics and ARGs
Treatment Method and System
| Citation: |



 Download:
Download:




