-
Cervical cancer ranks as the fourth most common malignancy and leading cause of cancer-related mortality among women globally, following breast, colorectal, and lung cancers (1-2). High-risk human papillomavirus (hrHPV) infection represents the primary risk factor for cervical cancer development (3). While 90% of HPV-infected individuals spontaneously clear the virus within 2 years, persistent infection occurs in specific populations, including those engaging in high-risk sexual behaviors, immunocompromised individuals, and those experiencing long-term recurrent HPV infections.
The pathogenesis of cervical cancer, initiated by hrHPV infection, is characterized by its low probability of occurrence and extended natural history, which poses significant challenges for clinical studies attempting to establish direct causal relationships between HPV vaccination, infection, and cervical cancer outcomes. Mathematical models serve as valuable tools for decision-makers when relevant data are limited and outcomes remain uncertain. Current mathematical approaches in HPV vaccination and cervical cancer research encompass both static progression models (Markov models) and dynamic progression models (transmission dynamics models). Transmission dynamics models demonstrate superior reliability in simulating viral transmission within dynamic populations and temporal changes in health status compared to their static counterparts (4). Details of the model studies reviewed in this paper are provided in Table 1.
Author Year District Model HPV Types Precautions Natural history of disease Sources Vaccine Screening CC CIN Diseases for men Korostil 2013 Australia SIS/SIR/SIRS HPV-16 NA NA NA NA NA [8] Wang 2019 China SEIR NA NA NA NA NA NA [9] Johnson 2012 Sweden SICR HPV-6/11/ 16/18/31/33/45/52/58 NA Yes Yes CIN1, CIN2, CIN3 NA [10] Zechmeister 2009 Austria SICR No Yes Yes Yes CIN1, CIN2, CIN3 NA [12] Owusu 2022 England SIRS HPV-6/11/ 16/18/31/33/45/52/58 4v, 9vHPV NA Yes CIN1, CIN2&3 Anal/head and neck/penile cancer [14] Elbasha 2007 United States SVIR HPV-6/11/16/18 4vHPV Yes Yes CIN1, CIN2&3 NA [15] Van De Velde 2012 Canada HPV-ADVISE HPV-6/11/ 16/18/31/33/45/52/58 2v, 4v, 9vHPV Yes Yes CIN1, CIN2, CIN3 Anal/head and neck cancer [16] De La Fuente 2019 Spanish SIRS HPV-6/11/16/18/31/33/45/52/58 4v, 9vHPV NA Yes CIN1, CIN2&3 Anal/mouth/head and neck/penile cancer [17] Chou 2022 Taiwan, China SIRS HPV-6/11/ 16/18/31/33/45/52/58 2v, 9vHPV NA Yes NA NA [18] Malik 2016 No SVIR-SIR HPV-6/11/ 16/18/31/33/45/52/58 2v, 4v, 9vHPV NA NA NA NA [19] Sharomi 2017 United States SVEIR NA 2v, 4v, 9vHPV NA Yes CIN NA [20] Laprise 2014 Canada HPV-ADVISE HPV-6/11/ 16/18/31/33/45/52/58 4vHPV Yes Yes CIN1, CIN2, CIN3 Cancer of the anus, penis and oropharynx [21] Prem 2023 Worldwide HSA/HPV-ADVISE/
HarvardHPV-6/11/ 16/18/31/33/45/52/58 9vHPV Yes Yes CIN1, CIN2, CIN3 Cancer of the anus, penis and oropharynx [22] Jit 2015 United Kingdom HPV-ADVISE HPV-6/11/ 16/18/31/33/45/52/58 2v, 4vHPV Yes Yes CIN1, CIN2, CIN3 Cancer of the anus, penis and oropharynx [23] Burger 2018 Ugandan Harvard-HPV HPV-16/18/31/33/45/52/58 No Yes Yes NA NA [24] Cheung 2023 Hong Kong SAR, China SVIR HPV-6/11/ 16/18/31/33/45/52/58 9vHPV Yes Yes CIN1, CIN2&3 Anal/mouth/head and neck/penile cancer [26] Simoens 2021 Belgium SVIR HPV-6/11/ 16/18/31/33/45/52/58 2v, 9vHPV NA Yes CIN Anal/head and neck cancer [27] Drolet 2021 India\
Vietnam\
Uganda\
NigeriaSVI HPV-6/11/ 16/18/31/33/45/52/58 2v, 4v, 9vHPV NA Yes CIN1, CIN2, CIN3 NA [28] Saldana 2022 Mexico SVAIC HPV-6/11/ 16/18/31/33/45/52/58 9vHPV NA Yes NA Anal/mouth/head and neck/penile cancer [29] Note: “NA” signifies that the corresponding section of the article has not been examined, whereas “No” denotes the absence of explanation within the article.
Abbreviation: HPV=human papillomavirus; NA=not applicable; SlS=susceptible-infected-susceptible; SlR=susceptible-infected-recovered; SlRS=susceptible-infected-recovered-susceptible; SElR=susceptible-exposed-infected-recovered; SlCR=susceptible-infected-cancer-recovered; SVR=susceptible-vaccinated-infected-recovered; HPV-ADVISE=human papillomavirus-ADvanced vaccination impact simulation model; SVlR-SlR=susceptible-vaccinated-infected-recovered-SIR; SVEIR=susceptilble-vaccinated-exposed-infected-recovered; HSA=health simulation model for HPV vaccination; CIN=cervical intraepithelial neoplasia; CC=cervical cancer.Table 1. Summary table of references.
The infectious disease transmission dynamics model, also termed the “compartmental model,” stratifies populations into distinct compartments based on disease occurrence and transmission patterns. Through differential equations, these models simulate and illustrate disease progression and epidemiological patterns, enabling analysis of epidemic causation, trend prediction, intervention assessment, and optimization of prevention and control strategies (5).
HPV transmission primarily occurs through heterosexual or homosexual intercourse, oral sex, or finger-vaginal contact (6). The virus demonstrates high infectivity, with over 50% of young women acquiring HPV infection within 4 years of sexual debut, and prevalence rates reaching 80% among sexually active female populations (7). Researchers have developed compartmental models to simulate, estimate, and refine critical parameters in the natural progression of HPV infection, providing evidence-based recommendations to policy-makers for HPV-associated cervical cancer prevention and control strategies.
Korostil and colleagues developed eight distinct compartmental models for HPV-16 by incorporating varying assumptions regarding the relationship between seropositivity and immunity (8). To account for the asymptomatic period characteristic of HPV infection, Wang et al. constructed a Susceptible-Exposed-Infected-Removed (SEIR) model and determined optimal vaccination strategies using a constrained time-varying approach (9). This SEIR model, offering greater biological accuracy than its SIR predecessor, has emerged as the predominant framework for simulating HPV transmission dynamics.
While these models effectively capture HPV infection dynamics, their predictive capacity is limited by the exclusion of disease progression states such as precancerous lesions and cancer. Johnson et al. addressed this limitation by developing a comprehensive transmission dynamics model encompassing the complete natural history from HPV infection through cervical cancer progression (10). The model structure is illustrated in Figure 1, where cervical intraepithelial neoplasia (CIN) stages are represented as CIN1 to 3. Following similar principles, Campos et al. and Zechmeister et al. constructed transmission dynamics models incorporating discrete compartments for CIN1–3, aligned with the disease’s natural progression (11–12).
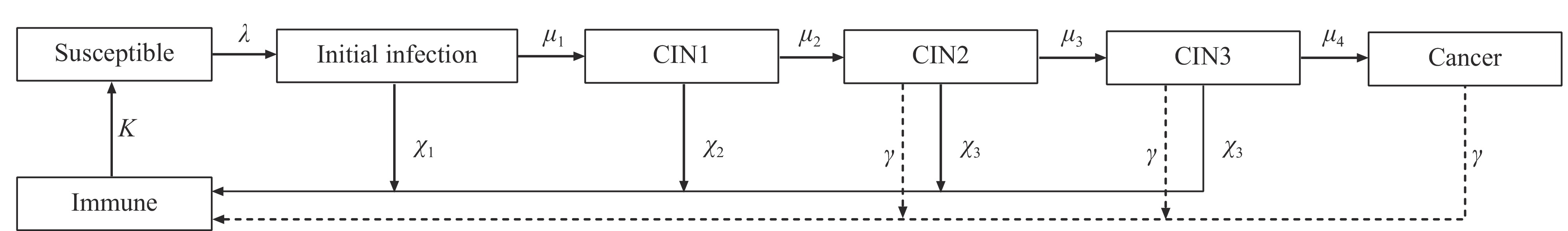 Figure 1.
Figure 1.Flowchart of the dynamics model.
Note: CIN1–3: cervical intraepithelial neoplasia stages are represented as CIN1 to CIN3.Vaccination represents the primary preventive measure against HPV infection and cervical cancer (13). Currently, three HPV vaccines are widely utilized: the bivalent (2vHPV), quadrivalent (4vHPV), and nine-valent (9vHPV) vaccines.
The introduction of the 9vHPV vaccine has enabled comprehensive comparative evaluations across vaccine types, considering factors such as immunological efficacy, coverage rates, and long-term benefits. Following the United Kingdom Department of Public Health’s 2021 decision to transition from quadrivalent to nine-valent vaccination, Kwame et al. (2022) (14) conducted a comparative analysis of 4vHPV and 9vHPV vaccines. They expanded upon Elbasha et al.’s original model (15) by incorporating additional compartments and differential equations to account for infections and diseases associated with HPV-31, -33, -45, -52, and -58. Their findings demonstrated that transitioning from 4vHPV to 9vHPV vaccination in national programs yields substantial health and economic benefits. Similar comparative analyses of 2vHPV/4vHPV versus 9vHPV vaccines in developed countries were conducted through transmission dynamics modeling by Nicolas et al. (16), Jesús et al. (17), Chou et al. (18), and Malik et al. (19). These studies, conducted in Canada, Spain, Taiwan, China, and the USA, corroborated Kwame’s findings (14), demonstrating superior reduction in HPV infection prevalence and cervical cancer cases with 9vHPV vaccination, along with enhanced cost-effectiveness.
The effectiveness and persistence of HPV vaccine protection correlate with dosing schedules. Sharomi et al. (2017) developed a deterministic model stratifying the female cohort by age (≤26 years and >26 years) and vaccination doses. Their analysis revealed that high vaccination adherence reduced HPV infections, with three-dose regimens demonstrating superior efficacy compared to two-dose or single-dose schedules (20).
In contrast to Sharomi’s findings regarding cost-effectiveness and immune protection duration, Laprise et al. (21) evaluated different dosing scenarios by comparing cumulative reduction percentages and ICERs of HPV-related cancers. Their analysis suggested that as two-dose vaccine protection duration increases, its cancer prevention efficacy approaches that of three-dose regimens, while demonstrating progressive cost-effectiveness advantages. Prem et al. integrated three established transmission dynamics models to evaluate HPV vaccine impact across varying doses, efficacy levels, protection duration, and coverage rates (22). All three models consistently predicted that a single-dose HPV vaccination program achieving 80% global coverage could provide robust population protection cost-effectively. Additional transmission dynamics analyses by Jit et al. (23) and Burger et al. (24) in the UK and US, respectively, demonstrated that single-dose HPV vaccination prevents nearly as many cervical cancer cases as multi-dose regimens, provided protection persists for 20 years or maintains at least 80% efficacy.
The World Health Organization’s updated HPV vaccination guidelines designate females aged 9–14 years as the primary target population. However, since the U.S. Food and Drug Administration’s initial approval of the HPV vaccine in 2006, the majority of the population has exceeded this recommended age range (25). Consequently, vaccination strategies have expanded beyond routine programs to address women outside the target age group through various age-specific approaches, including both routine and catch-up vaccination protocols. The guidelines also indicate that when resources permit, countries may extend vaccination to secondary populations, including males and older females.
Elbasha and colleagues developed a comprehensive compartmental model tracking disease progression from HPV infection to cervical cancer (15). Their analysis demonstrated that implementing 4vHPV vaccination for individuals ≤12 years of age, combined with catch-up vaccination for those aged 12–24 years across both sexes, yielded the most substantial reductions in condyloma acuminatum, CIN, and cervical cancer cases while maintaining cost-effectiveness. Chesson et al. adapted this model to Hong Kong’s context by incorporating local demographic data, sexual behavior patterns, and adjusting for cervical cancer detection parameters and screening rates (26). Their findings revealed that supplementing an intersex vaccination strategy with a catch-up program would enhance the reduction of HPV-associated diseases while remaining cost-effective. Similarly, Simoens et al. applied Elbasha’s model framework to evaluate the feasibility of implementing a two-sex 9vHPV vaccination strategy in Belgium (27). Their results corroborated previous literature, confirming the superior effectiveness of gender-neutral vaccination approaches compared to female-only strategies.
However, these models primarily reflect the contexts of developed nations, where vaccination strategy implementation is predominantly influenced by economic capacity, geographic accessibility, and healthcare insurance systems. Studies examining the expansion of HPV vaccination initiatives in resource-limited settings within developing and least developed nations have yielded markedly different conclusions.
Drolet et al. evaluated individualized vaccination strategies across four low- and middle-income countries (India, Vietnam, Uganda, and Nigeria) (28). Through comparative analysis of seven distinct HPV vaccination strategies, they determined that routine vaccination of 9–year-old girls, supplemented by vaccination of girls aged 9–14 years during the initial implementation year, represented the most efficient and cost-effective approach compared to no vaccination scenarios. Notably, male vaccination strategies emerged as the least efficient and cost-effective option, rendering them unsuitable for implementation in low- and middle-income regions. Similarly, Saldaña et al. developed a two-sex deterministic mathematical model in Mexico to evaluate four different HPV vaccination strategies (29). Their findings support a hierarchical vaccination approach in resource-constrained settings, prioritizing young girls first, followed by adult females, young boys, and adult males, respectively.
The prevention of cervical cancer fundamentally relies on understanding HPV transmission dynamics and implementing effective vaccination strategies. Dynamic transmission models have emerged as invaluable tools for analyzing viral spread patterns and evaluating vaccine impact. Evidence consistently demonstrates that the 9-valent HPV vaccine offers superior effectiveness and cost-efficiency compared to its bivalent and quadrivalent predecessors, particularly in developed nations. However, resource-limited settings benefit most from targeted strategies focusing on young girls and adult women. While extending vaccination coverage to male populations and older age groups can provide additional population-level benefits, such approaches may not always be economically or logistically feasible. These findings underscore the importance of developing context-specific vaccination strategies that prioritize coverage and accessibility within local resource constraints. Future research should address implementation challenges in low-resource regions and evaluate long-term strategies to achieve sustainable reductions in global cervical cancer burden.
HTML
| Citation: |



 Download:
Download:




