-
Glycidyl methacrylate (GMA) serves as a crucial industrial component in composite and epoxy polymer manufacturing and is classified as a high-volume chemical by the Organization for Economic Cooperation and Development screening information dataset (1). Following the publication of a 104-week inhalation carcinogenicity study in mice by the Japan Society for Occupational Health (JSOH) in 2015, JSOH (2) established an occupational exposure limit of 0.01 ppm for GMA (0.06 mg/m3, with conversion factors at 25 °C and 760 torr: 1 ppm=5.81 mg/m3; 1 mg/m3=0.172 ppm). Subsequently, in 2019, the International Agency for Research on Cancer (IARC) (2) classified GMA as a “probable human carcinogen” (Class 2A) based on this study and additional toxicological evidence. This classification prompted JSOH (3), the European Chemicals Agency (ECHA)(4), and the American Conference of Governmental Industrial Hygienists (5) to revise their respective occupational exposure limits (OELs) for GMA to 0.016 ppm and 0.01 ppm. In contrast, China’s current occupational exposure limit for GMA remains at a maximum permissible concentration of 5 mg/m3 (5), with no established time-weighted average concentration (PC-TWA). This standard has remained unchanged for over three decades, creating a significant disparity between Chinese regulations and those of Europe, the United States, and Japan.
The regulatory framework for OELs in China differs fundamentally from international standards, as Chinese standards are primarily mandatory rather than recommended guidelines. Given China’s specific industrial context, there is an urgent need to develop and revise the OELs for GMA to align with contemporary scientific understanding and international best practices.
The benchmark dose (BMD), introduced by Crump (6), represents the statistically derived lower confidence limit of the dose that produces a predetermined benchmark dose response (BMR, typically 1%–10%). This methodology was developed to address significant limitations inherent in the traditional no observed adverse effect level (NOAEL) approach. The scientific committee (SC) endorses the BMD approach as scientifically superior to the NOAEL method for determining a point of departure (PoD)(7). In 2017, the European Food Safety Authority (EFSA)(8) published updated guidance recommending model averaging as the preferred methodology for calculating BMD confidence intervals. The SC’s updated guidance reaffirms that the BMD approach, particularly model averaging, should be the primary method for deriving PoDs from critical dose-response data when establishing health-based guidance values and margins of exposure.
This investigation aims to establish scientifically robust OELs for GMA through the application of BMD analysis and Bayesian model averaging (BMA) techniques. Our methodology employs BMD analysis to identify potential toxic effect endpoints for GMA, selecting those with the lowest benchmark dose lower bound (BMDL10) as critical effects. To enhance the precision and reliability of GMA risk assessment, this study further refined these critical effect outcomes using BMA.
-
This investigation utilized data from a 104-week inhalation carcinogenicity study conducted in Japan. The experimental design comprised four groups: three treatment groups and one control group, with 50 female and 50 male rats per group, totaling 400 animals. Subjects were exposed to GMA via inhalation for 6 hours daily, 5 days weekly, throughout the 104-week period. The administered concentrations were 0 (control), 0.6, 2.5, and 10 ppm for both sexes. Detailed study information is accessible at https://anzeninfo.mhlw.go.jp/user/anzen/kag/pdf/gan/0795MAIN.pdf.
Given the absence of definitive human epidemiological evidence for GMA exposure, with existing case reports and occupational investigations lacking precise exposure quantification and being confounded by other potential sensitizing agents, these studies were deemed unsuitable for OEL determination. The selected study adhered to Good Laboratory Practice standards, featured appropriate duration, and employed the relevant exposure route (inhalation), as acknowledged in IARC’s assessment. Furthermore, this study serves as the foundational evidence for GMA OELs established by JSOH, ECHA, and other regulatory bodies, validating its selection as the primary toxicological evidence for establishing GMA OELs.
For OEL assessment, chronic toxicity and carcinogenicity were identified as the primary critical effects of GMA. The study results were systematically analyzed, incorporating various uncertainty factors and categorizing endpoints into non-neoplastic and neoplastic lesions, with stratification by sex. Carcinogenicity outcomes were classified by overall and terminal rates, all of which were incorporated into the BMD analysis. Only endpoints demonstrating statistical significance (P<0.05) were included in the analysis.
-
The BMD methodology employs statistical models to estimate toxic response probabilities at specified doses, facilitating the identification of dose-response relationships and determination of lower safe doses. Using BMDS software (version 3.3.2, EPA, the United States), this study analyzed statistically significant endpoints (P≤0.05) using nine models: Dichotomous Hill, Gamma, Log-Logistic, Multistage Degree, Weibull, Logistic, Log-Probit, Probit, and Quantal Linear. The BMR level was set at 0.1, with a confidence level of 0.95. The endpoint yielding the lowest BMDL10 was selected as the BMD for GMA. Model fit assessment incorporated goodness-of-fit analysis, statistical testing, residual analysis, Akaike information criterion (AIC) value evaluation, P value examination, and nested testing.
-
This study utilized BMDS software for modeling key effects. The software assigns prior probabilities to each model based on model selection criteria, with equal default weights assigned to all models. Using binomial sampling for dichotomous endpoints and Normal or Lognormal distributions for continuous data, the software employs Laplace approximation to correct prior density. It then performs BMD estimation through maximum a posteriori probability estimation, computes posterior probabilities across multiple models, and assigns differential weights for model averaging calculations. Bayesian model averaging enhances estimation accuracy by combining results from multiple models. The incorporation of prior information substantially reduces BMD estimation uncertainty and prevents the selection of extreme models that might occur when relying solely on AIC values. By comprehensively evaluating all candidate models, this approach minimizes model selection bias, thereby improving result accuracy and calculation reliability (9).
A PoD represents the dose at which an adverse effect manifests following specific exposure, whether determined empirically or through dose-response modeling (10). In our analysis, we employed BMA to assign differential weights across models for averaging calculations and selected the BMDL10 as the PoD, following EFSA and EPA recommendations for quantal data.
-
Our analysis incorporated uncertainty factors (UFs) for interspecies and intraspecies differences, along with effect severity. This study applied an interspecies factor of 2.5 and, following ECHA recommendations, an intraspecies factor of 5 for worker populations (compared to 10 for general populations) when establishing derived ineffective response levels. For GMA-induced non-neoplastic lesions, which are reversible, this study applied an uncertainty factor of 1. However, given the severity of GMA-induced neoplastic lesions, this study implemented an uncertainty factor of 10.
The final PoD serves as the basis for OEL calculation. Using equal prior probabilities for all models, this study derived GMA OEL values using the following equation:
$$ OEL=PoD/UFs $$ where, OEL means occupational exposure limit (ppm); PoD means point of departure; UFs means uncertainty factors.
-
Analysis of non-neoplastic lesions in male mice revealed 10 endpoints with BMDL10 values ranging from 0.100 to 8.157 ppm. The respiratory metaplasia of the nasal cavity olfactory epithelium yielded the lowest BMDL (0.103 ppm), with optimal fit achieved using the Log-Probit model. In female mice, nine non-neoplastic endpoints produced BMDL10 values between 0.077 and 6.825 ppm, with nasopharyngeal eosinophilic change showing the lowest BMDL10 (0.077 ppm) using the Dichotomous Hill model. Detailed BMD information is presented in Table 1.
Endpoints Sex BMR Recommended
modelP AIC BMD
(ppm)BMDL10
(ppm)Death Male 0.1 Log-logistic 0.243 265.728 0.964 0.441 Female 0.1 − <0.1 − − − Nasal cavity angiectasis Male 0.1 Quantal linear 0.968 34.937 16.821 8.157 Female 0.1 Weibull 1.000 42.496 9.803 6.825 Nasal cavity eosinophilic change: olfactory epithelium Male 0.1 Log-logistic 0.354 174.269 3.732 2.000 Female 0.1 Weibull 0.485 221.871 8.783 2.561 Nasal cavity eosinophilic change: respiratory epithelium Male 0.1 Log-probit 0.578 218.022 1.308 0.435 Female 0.1 − <0.1 − − − Nasal cavity respiratory metaplasia: olfactory epithelium Male 0.1 Log-probit 0.704 189.927 0.256 0.103 Female 0.1 − <0.1 − − − Nasal cavity hyperplasia: transitional epithelium Male 0.1 Multistage degree 3 0.977 86.385 4.505 3.048 Female Quantal linear 0.908 49.388 9.380 5.338 Nasal cavity regeneration: respiratory epithelium Male 0.1 Multistage degree 1 0.730 96.060 3.1808 2.232 Female 0.1 Gamma 1.000 121.602 1.929 1.166 Nasopharynx eosinophilic change Male 0.1 Weibull 0.466 141.740 9.471 2.763 Female 0.1 Dichotomous hill 0.224 227.750 0.294 0.077 Nasal cavity inflammation: respiration epithelium Male 0.1 Multistage degree 2 0.994 67.035 6.171 4.340 Female 0.1 − <0.1 − − − Nasal cavity respiratory metaplasia: gland Male 0.1 Multistage degree 2 0.819 195.185 0.261 0.194 Female 0.1 − <0.1 − − − Nasal cavity squamous cell metaplasia: respiratory epithelium Male 0.1 − <0.1 − − − Female 0.1 Weibull 1.000 61.295 9.388 5.310 Nasa cavity necrosis: olfactory epithelium Male 0.1 − <0.1 − − − Female 0.1 Weibull 0.365 52.707 9.921 6.430 Uterus nodule Female 0.1 Multistage degree 3 0.968 222.173 8.403 3.053 Ovary enlarged Female 0.1 Log-probit 0.334 127.124 7.082 2.002 Note: Maximum multistage degree is 3. “-”: due to the goodness-of-fit P<0.1, the models are poorly fitted and we do not recommend any of them.
Abbreviation: BMR=benchmark response; AIC=akaike information criterion; BMD=benchmark dose; BMDL=benchmark dose lower confidence limit.Table 1. Benchmark dose analysis results of non-neoplastic lesions in mice.
Analysis of tumorigenic endpoints revealed 16 distinct endpoints in male mice, with BMDL10 values ranging from 0.756 to 10.197 ppm. The terminal rate of nasal cavity hemangioma demonstrated the lowest BMDL10, best fitted by the Dichotomous Hill model. Female mice exhibited 16 neoplastic endpoints with BMDL10 values ranging from 0.791 to 7.434 ppm, with uterine histiocytic sarcoma showing the lowest BMDL10, optimally fitted using the Log-Logistic model. Table 2 summarizes these findings, which identify the respiratory system as the primary target organ for GMA toxicity in female rats.
Site Tumor Overall rates Terminal rates Recommended model BMDL10 AIC Recommended model BMDL10 AIC Male Nasal cavity Adenoma Weibull 9.119 24.697 Weibull 4.612 13.483 Hemangioma Dichotomous hill 1.437 72.664 Dichotomous hill 0.756 34.532 Hemangiosarcoma Log-probit 4.029 63.888 Weibull 10.197 7.205 Hemangioma, hemangiosarcoma Multistage degree 1 2.101 4.815 Dichotomous hill 0.841 36.029 Hemangioma, hemangiosarcoma,
adenomaLog-probit 2.023 98.715 Quantal linear 1.069 35.197 Lung Bronchiolar-alveolar adenoma Weibull 7.626 97.267 Weibull 3.241 45.252 Stomach Squamous cell papilloma Weibull 9.245 38.711 Weibull 4.612 13.483 Harderian gland Adenoma Log-logistic 4.457 91.079 Log-logistic 1.150 42.070 Female Nasal cavity Hemangioma Dichotomous hill 1.129 69.193 Multistage degree 1 1.649 22.193 Hemangiosarcoma Quantal linear 6.929 40.167 − − − Hemangioma, hemangiosarcoma Multistage degree 3 2.797 84.368 Multistage degree 1 1.649 22.193 Hemangiosarcoma, adenocarcinoma Quantal linear 6.029 44.961 Weibull 4.947 8.279 Adenocarcinoma, hemangioma,
hemangiosarcomaMultistage degree 1 2.629 86.773 Weibull 3.973 9.535 Lung Bronchiolar-alveolar carcinoma Weibull 7.092 55.751 Weibull 3.973 9.534 Bronchiolar-alveolar adenoma,
bronchiolar-alveolar carcinomaWeibull 7.434 10.416 Log-logistic 1.447 47.627 Uterus Histiocytic sarcoma Logistic 3.448 227.296 Log-logistic 0.791 2.140 Harderian gland Adenoma Log-logistic 6.704 68.333 − − − Note: Overall rates represent the number of tumor-bearing animals relative to total animals examined at the site. Terminal rates indicate tumor incidence at terminal kill. Maximum multistage degree is 3. “−”: due to the goodness-of-fit P<0.1, the models are poorly fitted and we do not recommend any of them.
Abbreviation: AIC=akaike information criterion; BMDL=benchmark dose lower confidence limit.Table 2. Benchmark dose analysis results of neoplastic lesions in mice.
-
Figure 1 illustrates the dose-response relationships derived from Bayesian model averaging across different endpoints. Table 3 presents the posterior probabilities and BMD values post-model averaging. The model averaging approach, which incorporated all viable alternative models while excluding extreme cases, yielded more robust results than classical single-model analysis. For male rats’ olfactory epithelial nasal cavity respiratory metaplasia, the Multistage, Quantal Linear, and Weibull models demonstrated superior fit and significantly influenced BMDL10 calculations, receiving greater computational weight and yielding a model-averaged BMDL10 of 0.118 ppm. Female rats’ nasopharyngeal eosinophilia change was best characterized by the Multistage, Quantal Linear, and Log-Logistic models, producing a BMDL10 of 0.157 ppm. For mice carcinogenicity endpoints, the Probit, Multistage, and Quantal Linear models provided optimal fit, yielding BMDL10 values of 1.733 and 1.081 ppm for males and females, respectively. The lower PoD for non-carcinogenic effects compared to carcinogenic effects indicates that intranasal lesions represent the most sensitive endpoint for GMA inhalation exposure. Application of UFs to the model-averaged results produced OEL values of 0.0094, 0.0126, 0.0139, and 0.0086 ppm, aligning with established limits in the EU (0.016 ppm), Japan (0.012 ppm), and the US (0.01 ppm). Current evidence supports 0.01 ppm as a protective PC-TWA for occupational GMA exposure.
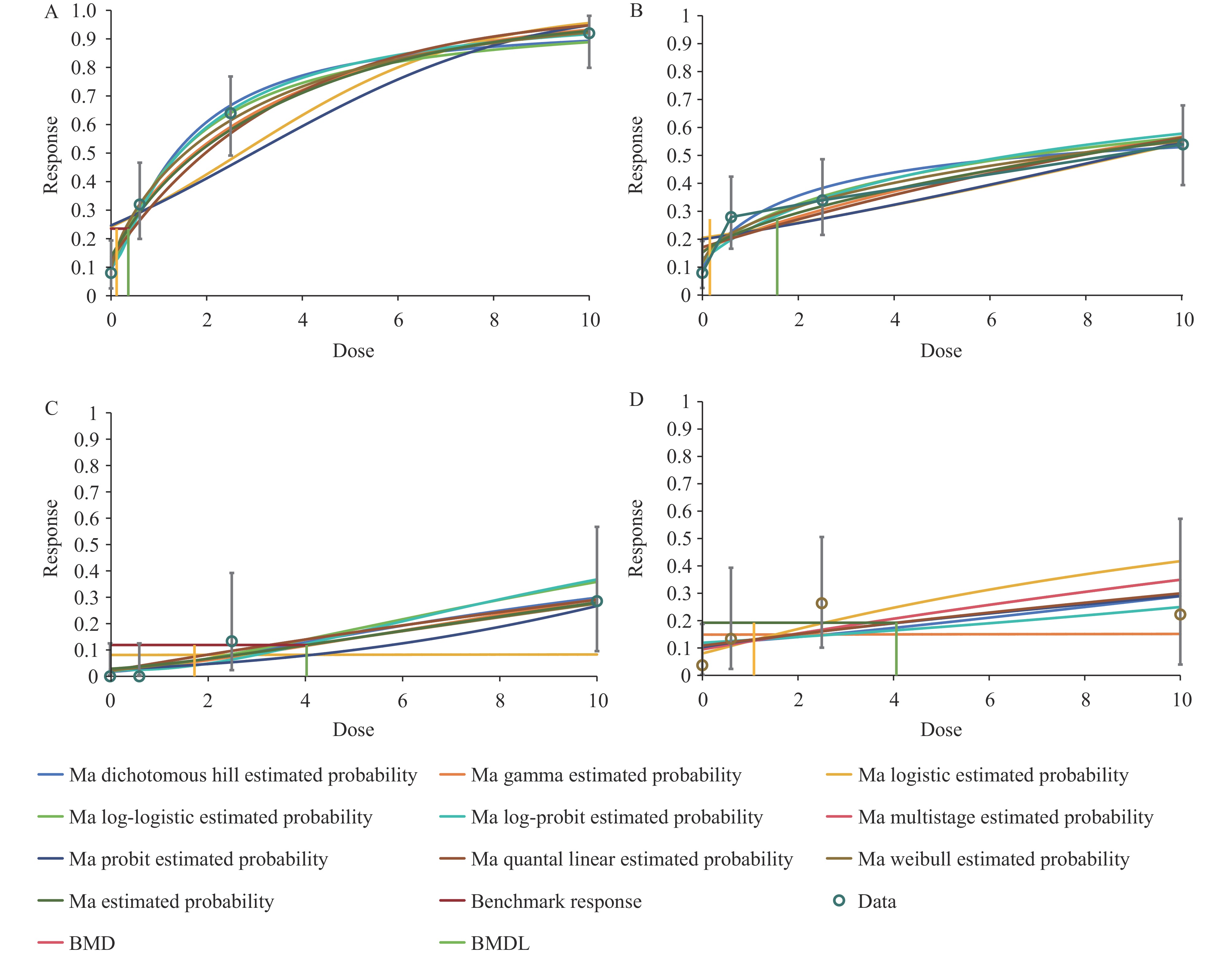 Figure 1.
Figure 1.Dose-response relationships of bayesian modeling average. (A) Male mice nasal cavity respiratory metaplasia: olfactory epithelium. (B) Female mice Nasopharynx eosinophilic change. (C) Male mice nasal cavity hemangioma. (D) Female mice uterus histiocytic sarcoma.
Abbreviation: Ma=model average; BMD=benchmark dose; BMDL=benchmark dose lower confidence limit.Model Male mice nasal cavity respiratory metaplasia: olfactory epithelium Female mice nasopharynx eosinophilic change Male mice nasal cavity hemangioma Female mice uterus histiocytic sarcoma Posterior
probabilityBMDL10 Posterior
probabilityBMDL10 Posterior
probabilityBMDL10 Posterior
probabilityBMDL10 Dichotomous hill 0.035 0.119 0.063 0.076 0.155 1.398 − − Gamma 0.030 0.064 0.026 0.290 0.061 2.034 0.068 1.248 Logistic 0 0.709 0.041 2.161 0.001 3.714 0.034 6.380 Log-logistic 0.043 0.096 0.092 0.091 0.076 1.575 0.065 0.431 Log-probit 0.023 0.146 0.007 0.176 0.024 1.964 − − Multistage 0.391 0.289 0.318 1.112 0.204 1.760 0.274 1.403 Probit 0 0.796 0.061 2.072 0.219 3.994 0.206 3.185 Quantal linear 0.391 0.289 0.318 1.112 0.204 1.760 0.274 1.403 Weibull 0.087 0.053 0.074 0.068 0.057 4.098 0.080 0.518 Model average − 0.118 − 0.157 − 1.733 − 1.081 OELs value − 0.0094 − 0.0126 − 0.0139 − 0.0086 Note: Maximum multistage degree is 3. “−”: Model not fitted or no data.
Abbreviation: BMDL=benchmark dose lower confidence limit.Table 3. Bayesian model averaging results for minimal effect endpoints in mice benchmark dose analysis.
-
This study employed BMD analysis and BMA for GMA risk assessment, utilizing animal studies to identify primary sites of toxic effects. The results demonstrated that GMA’s principal adverse effects manifest at initial exposure sites, specifically the foregut following oral exposure and respiratory tract after inhalation exposure. Chronic GMA exposure in mice induced carcinogenic effects, evidenced by increased tumor incidence in multiple sites including the nasal cavity, lungs, stomach, and uterus. Animal studies (3) have also established GMA’s reproductive toxicity, while several case reports (11-12) have documented allergic reactions in humans exposed to GMA.
The BMD approach demonstrates superior sensitivity compared to the NOAELs/LOAELs methodology, ensuring comprehensive identification of potentially sensitive endpoints for risk assessment. Our analysis yielded BMDL10 values of 0.103 and 0.077 ppm as general toxicity PoDs, substantially lower than the NOAEL/LOAEL-derived PoD (0.6 ppm). These results produced OEL values (0.01 ppm) slightly below ECHA’s 8h-TWA (0.016 ppm) and JSOH’s OEL-M (0.012 ppm). The BMD approach offers distinct advantages: it transcends experimental dose limitations, shows reduced sensitivity to dose spacing, and incorporates both dose-response curve characteristics and statistical uncertainties from data quality. When statistical power is constrained by limited data points or high variability, the BMD approach provides more robust conclusions by considering the complete dose-response curve and addressing statistical limitations more effectively than NOAEL. Consequently, our derived PoD incorporates more comprehensive information and better reflects GMA’s actual toxic effect profile. The alignment of final OEL values, despite different methodological approaches (ECHA and JSOH applying a 10-fold uncertainty factor for LOAEL to NOAEL extrapolation), validates 0.01 ppm as a reasonable PC-TWA for GMA under current evidence.
The BMA methodology employed here utilizes a comprehensive dose-response model with weighted mathematical components to calculate model means, generating reliable estimates and confidence intervals. This approach leverages prior information to enhance parameter estimation precision while accounting for model uncertainty. Traditional single-model statistical approaches risk introducing “model selection error (13),” which can be mitigated through model averaging techniques (14). The method’s inherent capacity to address model uncertainty provides enhanced flexibility and reliability in both model selection and parameter estimation (9), ultimately establishing a more robust foundation for risk quantification (7).
This study was subject to some limitations. The selection of appropriate prior distributions for BMA in BMDS software presents challenges, particularly without comprehensive background knowledge. Furthermore, our reliance on animal test data for OEL recommendations may not fully reflect actual plant operational conditions, necessitating additional field studies for developing more practical OELs.
Our recommended PC-TWA of 0.01 ppm for GMA represents a conservative approach to worker protection. According to ECHA’s (4) T25 methodology dose-response relationship, this concentration corresponds to approximately 40 additional cancer cases per 100,000 exposed workers. Given GMA’s demonstrated high sensitization potential in animal tests and case reports, dermal exposure remains an important area for future research. While modern closed-system manufacturing processes under controlled conditions (2) facilitate maintaining low workplace GMA concentrations, current national OELs require revision. Specifically, establishing PC-TWA and revising maximum allowable concentration are crucial steps toward enhanced worker health protection.
HTML
Dataset and Endpoint Selection
BMD Modeling
BMA and Determining PoD
Application of Uncertainty Factors and Calculation of OEL
BMD Analysis Results
BMA Results
| Citation: |



 Download:
Download:





