-
Pertussis, commonly known as whooping cough, is attributable to the Gram-negative bacterium Bordetella pertussis. Vaccination options against B. pertussis include whole-cell pertussis (wP) and acellular pertussis (aP) vaccines. Acellular vaccines typically consist of purified proteins from B. pertussis, especially filamentous hemagglutinin (Fha), pertussis toxin (Ptx), and pertactin (Prn), and often include fimbrial proteins (Fim2 and Fim3). During the 1990s, several countries recommended substituting the less reactive aP vaccines for wP vaccines (1). In China, the National Immunization Program has been administering the diphtheria–tetanus–wP vaccine since the 1980s. A shift to the diphtheria–tetanus–aP (DTaP) vaccine, which incorporates Ptx, Prn and Fha as bioactive components, occurred between 2007 and 2013 (2).
The resurgence of pertussis poses a significant global public health challenge, primarily due to vaccine escape and antigenic shifts in Bordetella pertussis. There has been a noted divergence in the antigens of B. pertussis between the strains circulating in the population and those present in vaccines (3–4). Strains exhibiting a novel promoter for Ptx (ptxP3) have been identified in various countries, including China (5–7). Significant virulence factors such as Prn and Fha, both integral components of B. pertussis vaccines, have shown variations. The first report of Prn-deficient B. pertussis strains occurred in the USA in 1994 (4), with similar strains later identified in China in 2019 (8). Instances of Fha-deficient B. pertussis strains have also been documented. Additionally, there has been a rise in the occurrence of high-level macrolide-resistant B. pertussis strains, particularly in China since 2013, which is linked to the A2037G mutation in the 23S rRNA gene (5–6).
Since 2022, two Beijing-based sentinel hospitals have implemented a pertussis surveillance study, monitoring cases suspected of pertussis and conducting laboratory tests for B. pertussis using real-time PCR from January 2022 to December 2023. A total of 44 B. pertussis strains were isolated from nasopharyngeal swab specimens collected from 44 outpatients, consisting of 13 infants, 28 children, and 3 adults. These strains underwent genetic analysis through sequencing using the Illumina HiSeq 2500 system. This analysis focused on genes encoding eight vaccine-related antigens (ptxA, ptxC, ptxP3, prn, fim2, fim3, fhaB, and tcfA) and a type III secretion system gene (bscI), analyzed using BLAST with an E-value threshold of 1e−5. Allelic variations for ptxA, ptxC, ptxP3, prn, fim2-1, fim3-1, fhaB, tcfA2, and bscI were identified through comparison on the BIGSdb-Pasteur platform (https://bigsdb.pasteur.fr/cgi-bin/bigsdb/bigsdb.pl?db=pubmlst_bordetella_seqdef). All identified Bordetella pertussis strains shared the same antigenic profile: ptxA1, ptxC2, ptxP3, prn150, fim2-1, fim3-1, fhaB1, tcfA2, and bscI2. Notably, 14 strains had the prn150 allele located on the same DNA sequence contig, whereas in the remaining 30 strains, this allele was split across two contigs due to a gene disruption at a site 240 bp upstream from the prn start site, identified as a common Prn-deficiency mechanism (4). This disruption was characterized by a 6-bp (GCTAGA) overlap and was associated with a reversed insertion of IS481, hinted by a “CTAG” termination sequence (accession no. M22031)(Fig. 1). This insertion's location and orientation were confirmed by third-generation sequencing for two strains, one with an intact prn150 (BJSY2023BRK008) and one with a truncated prn150 (BJSY2022BRK001), performed on the PacBio platform. The completed genome of BJSY2022BRK001 confirmed reversed IS481 insertion in prn (Fig. 2). Additionally, two strains showed a Fha deficiency, evidenced by a deletion of a “G” at position 1087 in the homopolymeric G-tract of fhaB, a mutation previously documented for causing this deficiency (3).
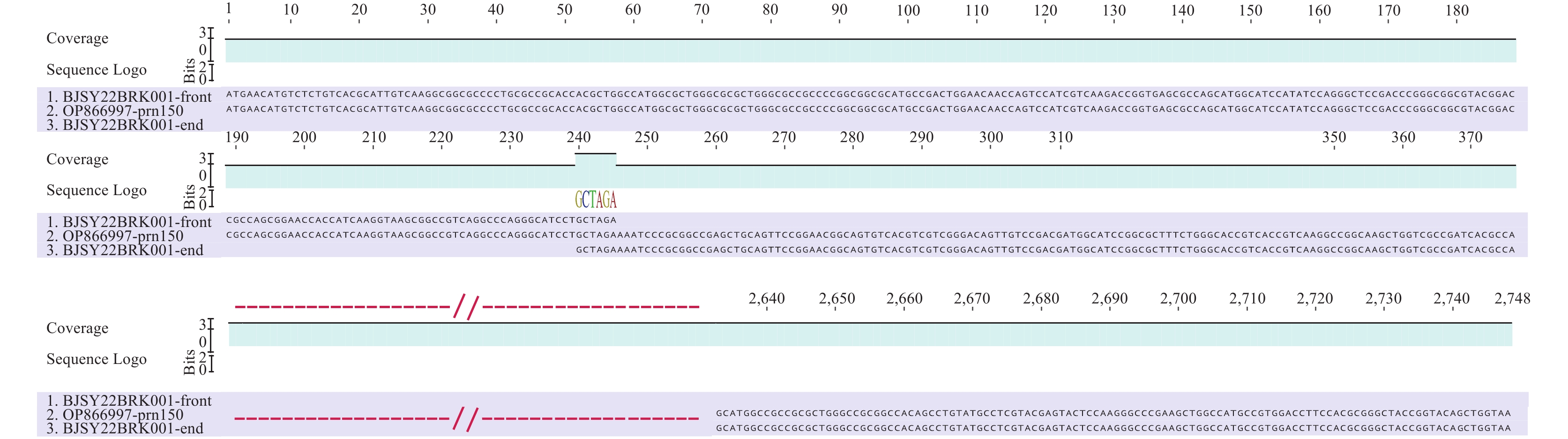 Figure 1.
Figure 1.Comparison of the disrupted prn gene with the complete prn150 allele, highlighting six overlapping bases (GCTAGA) between the contigs. OP866997-prn150: intact prn150.
 Figure 2.
Figure 2.Circularized genomes featuring complete and truncated prn150 genes. The purple ring denoted BJSY2023BRK008, while the light blue ring signified BJSY2022BRK001. The diagram illustrated the gene structures of complete prn150 and prn150 with a reversed IS481 insertion.
All Bordetella pertussis strains possessed identical 23S rRNA gene sequences and exhibited an A2037G mutation. Susceptibility testing conducted with E-test strips indicated that all strains were resistant to erythromycin and azithromycin, with minimum inhibitory concentrations (MIC) exceeding 256 μg/mL.
A phylogenetic tree was constructed based on core SNPs from 148 B. pertussis genomes. This set includes genomes from all strains analyzed in this study, two reference genomes (Tohama I, NC002929.2 and CS, CP086368), and an additional 102 genomes sourced from the NCBI database (BioProject no. PRJNA908268). The analysis revealed no specific linkage among the 44 isolated strains (Figure 3).
 Figure 3.
Figure 3.Phylogenetic tree based on core SNPs from Bordetella pertussis. The genome highlighted with a light blue background was sequenced in this study, and red fonts were Prn-deficient strains. Genomes marked with a red and green hexagon represented vaccine and Fha-deficient strains, respectively. The strains isolated during this study were distributed across various clusters within the tree.
Abbreviation: SNP=Single Nucleotide Polymorphism. -
Pertussis, a disease preventable by vaccination, has seen an increase in global incidence even with high vaccination coverage; for instance, coverage for the diphtheria-tetanus-pertussis (DTaP) vaccine in China is reported at 99% (7). The DTaP vaccines used in China comprise Bordetella pertussis strains with the antigenic composition of ptxA2/ptxC1/ptxP1/prn1/fim2-1/fim3-1/fhaB1/tcfA2. Recent studies have indicated shifts in the primary vaccine antigens of B. pertussis compared to the vaccine strains. Li et al. identified the predominant virulence-associated genotype in northern China as ptxA1/ptxC1/ptxP1/prn1/fim2-1/fim3A/tcfA2, noting a rare occurrence of the ptxP3 strains, all of which were sensitive to erythromycin (6). Fu et al. discovered that 41.1% of the highly virulent B. pertussis strains carried ptxP3/prn2/ptxC2 and were all susceptible to macrolides, whereas the remaining 58.9% were less virulent, carrying ptxP1/prn1/ptxC1 (5). All identified strains displayed the antigenic configuration ptxA1/ptxC2/ptxP3/prn150/fim2-1/fim3-1/fhaB1/tcfA2 and bscI2, markedly diverging from the vaccine strain. Notably, all isolated strains contained ptxP3/bscI2, differing from previous studies that associated ptxP3 strains with bscI3 (9). Strains with ptxP3 are reported to produce more Ptx than those with ptxP1, potentially indicating increased virulence (10). However, there are differing opinions on whether ptxP1 strains might cause more severe diseases than ptxP3 strains (5). Further research is necessary to elucidate the mechanisms driving the rapid proliferation of ptxP3 strains in recent years.
Various mechanisms can result in Prn deficiency, such as mutations, deletions, and insertions within the prn gene. Cai et al. observed Prn-deficient strains in 11.7% of samples from Shanghai, characterized by nucleotide deletions and stop codon mutations in prn (7). The insertion of IS481 was identified as the predominant cause of prn disruption (4). Another study reported that 100% of Prn-deficient isolates had Prn inactivated by IS481 insertion, which occurred independently across different B. pertussis lineages (4). In our research, we detected a high prevalence (68.2%, 30/44) of Prn-deficient B. pertussis strains resulting from IS481 insertion, representing the first documentation of such a significant occurrence.
Fha is a significant virulence factor of B. pertussis. In this study, we identified two strains deficient in Fha and one strain that was deficient in both Prn and Fha, marking the first reported cases of Fha-deficient strains in China. The emergence of strains with deficiencies in multiple immunogens poses an increasing public health concern, highlighting the necessity for ongoing surveillance of B. pertussis strains in China.
All B. pertussis strains examined in this study displayed resistance to macrolides, aligning with results from a recent study (7). The increasing prevalence of macrolide-resistant B. pertussis strains presents considerable challenges to clinical treatment.
The increasing dispersion of ptxP3 strains, which are potentially more virulent and exhibit higher resistance to macrolides, poses substantial challenges for treating pertussis. Given the widespread prevalence of Prn-deficiency and the emergence of Fha-deficient B. pertussis strains, the development of vaccines by numerous local manufacturers is complicated, as Prn and Fha are key antigens in these vaccines. Selecting the appropriate pertussis vaccine for inclusion in China’s National Immunization Program is crucial to enhancing the efficacy of pertussis prevention and control strategies.
The analysis in this study encompassed a limited number of strains, underscoring the need for ongoing surveillance of pertussis pathogens. In addition, only the genotypes of vaccine-associated antigens of isolated strains were analyzed in this study. The subsequent research on the function and pathogenic mechanism of antigen proteins is crucial for the evaluation of vaccine effectiveness and the development of vaccines.
-
No conflicts of interest.
-
The staff of the participating hospitals and colleagues at Shunyi CDC.
HTML
| Citation: |




 Download:
Download:




