-
We highlight the paramount importance of pretreatment processes preceding the biological treatment of antibiotic manufacturing wastewater as a strategy to curb the escalation of antibiotic resistance. In an effort to curtail the advancement of antimicrobial resistance (AMR) in antibiotic production in China, a comprehensive array of technical and regulatory initiatives have been implemented. These measures offer valuable insights that could inform and enhance global AMR containment strategies. Looking ahead, there is a pressing need to form an interdisciplinary team tasked with refining antibiotic emission standards and tailoring environmental engineering practices for the pharmaceutical sector.
-
Antibiotic contamination is a pervasive environmental issue, largely stemming from the excretions of humans and livestock (urine and feces) as well as the effluent from antibiotic production processes. The pharmaceutical sector is notably significant in this regard, as its contributions to antibiotic pollution greatly exceed those of other sources. It is a critical nexus for the proliferation of antibiotic resistance (Figure 1). The production of active pharmaceutical ingredient (API) for fermentative antibiotics generally involves microbial fermentation, with typical concentrations expressed in milligrams per kilogram (mg/kg). Treating the resulting wastewater requires a biological process, given its high organic matter content. However, residual antibiotics in the wastewater can disrupt the microbial communities and promote an increase in antibiotic resistance genes (ARGs) within biological treatment systems (1–3). Consequently, this leads to the release of both antibiotics and ARGs into the surrounding environment. Adopting a One Health approach, effectively curbing AMR emissions from the pharmaceutical industry could lessen the environmental selection pressure on ARGs. In concert with reducing their transfer to humans and animals, this strategy offers a considerable advantage.
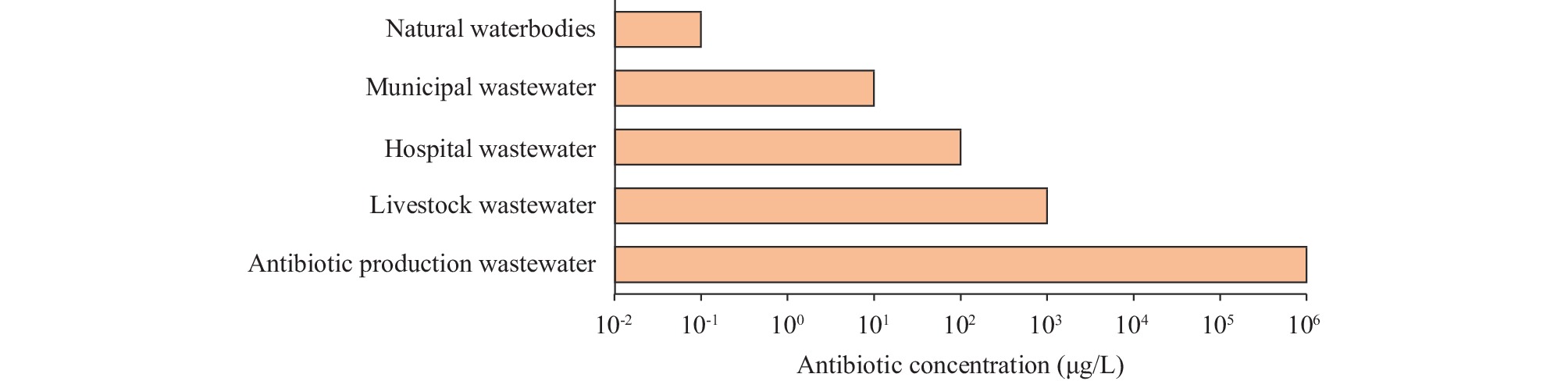 Figure 1.
Figure 1.Antibiotic pollution in the environment.
Note: This figure was modified from Larsson et al. (2021) (12), depicting a simplification of the typical ranges of antibiotic concentrations in various environmental media. -
Intergovernmental organizations are increasingly focused on the control of AMR stemming from pharmaceutical sector activities. In August 2013, the United Nations Environment Programme (UNEP) and the World Health Organization (WHO) teamed up to hold a symposium titled “Green Procurement for Pharmaceutical Manufacturing” in Bonn, Germany. The objective of this forum was to explore the viability and possible breadth of guidelines for environmentally responsible procurement practices within the healthcare field. During this gathering, attendees recognized an urgent need to broaden the conventional approach to evaluating pharmaceuticals and medical products. Traditionally, clinical decision-making relies on evidence-based standards, but the environmental impact of these products demands a wider view. A more comprehensive approach, factoring in a full range of environmental repercussions and life-cycle assessments, was proposed. Moving forward, it is expected that global procurement in the health sector will increasingly scrutinize pharmaceutical companies' responses to the AMR crisis. It will underscore the importance of choosing products that not only meet clinical requirements but are also designed and manufactured with environmental sustainability in mind (4).
In 2020, the WHO, in partnership with the Food and Agriculture Organization of the United Nations (FAO) and the World Organization for Animal Health (WOAH), published a significant report entitled “Technical brief on water, sanitation, hygiene and wastewater management to prevent infections and reduce the spread of antimicrobial resistance” (5). A prominent section within this brief focused on the “Manufacturing of antimicrobials”, highlighting the urgent need for pollution control measures in the production of antimicrobials and API. These measures are imperative for mitigating the global threat of AMR that arises from manufacturing processes. The WHO is currently developing new guidance titled “WHO Guidance on waste and wastewater management in pharmaceutical manufacturing with emphasis on antimicrobial production”, which will set forth pivotal criteria in this domain.
The pharmaceutical sector and its international supply chain have acknowledged the AMR risks associated with antibiotic production processes. The AMR Industry Alliance (6) is a collaboration of biotech, diagnostics, generics, and research-based pharmaceutical organizations, dedicated to driving and tracking industry advancements in combating AMR. In 2018, the AMR Industry Alliance’s set of antibiotic manufacturing discharge targets, based on Predicted No-Effect Concentrations (PNECs), was embraced by the Pharmaceutical Supply Chain Initiative (PSCI) (7). These targets offer a scientific framework for establishing environmentally safe concentrations of antibiotics released from manufacturing sites (8-9). More recently, in 2022, the AMR Industry Alliance, in conjunction with the British Standards Institution, unveiled the “Antibiotic Manufacturing Standard: Minimizing the risk of developing antibiotic resistance and aquatic ecotoxicity in the environment resulting from the manufacturing of human antibiotics” (10). This new standard specifies requirements aimed at diminishing the progression of AMR and mitigating associated ecological risks in surface waters due to antibiotic manufacturing activities.
It is important to recognize that the antibiotic manufacturing discharge benchmarks established by the AMR Industry Alliance are predicated on PNECs of antibiotics. These PNEC values are obtained through modeling techniques that specifically target pathogenic bacteria found in existing databases (11). While the current methodology provides a foundational approach, the veracity of the model would benefit from additional validation via empirical field studies. Moreover, although the PNECs are tailored to aquatic environments, there exists a significant challenge in extrapolating these effect levels to solid or semi-solid matrices (12-13). This limitation underscores the need for further research to adapt PNEC values for comprehensive environmental assessments.
-
The prevalent approach to managing wastewater from antibiotic manufacturing typically involves biological treatment systems due to its characterization as high-concentration organic wastewater (Figure 2). Biological treatment is a favored option in pharmaceutical operations as it can effectively reduce the levels of conventional pollutants such as chemical oxygen demand (COD), at a relatively low cost. However, antibiotics that persist in the wastewater also enter these biological treatment systems, imposing a selective pressure on the bacterial communities therein. This can lead to the discharge of both antibiotics and ARGs into the environment (14-15). To prevent excessive antibiotics from entering both the biological wastewater treatment system and the surrounding ecosystem, it is crucial to implement pretreatment strategies aimed at eliminating the antibacterial potency of the untreated antibiotic production wastewater. Therefore, the potential for physico-chemical pretreatment processes in dealing with these wastewaters warrants further investigation.
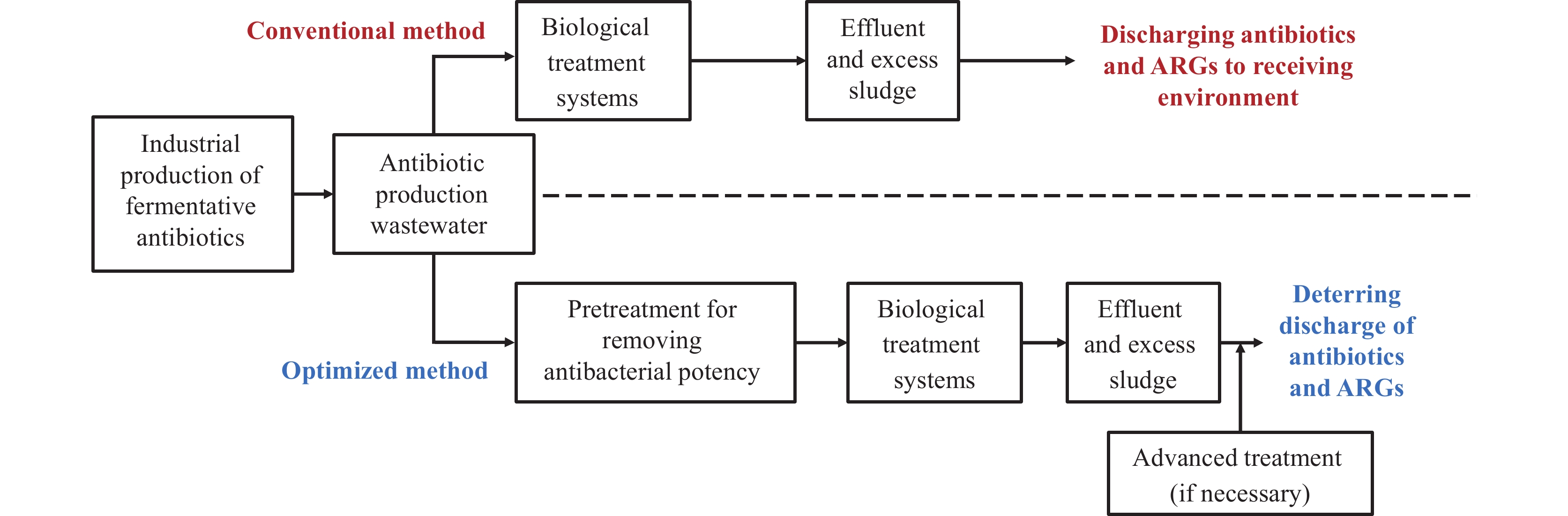 Figure 2.
Figure 2.Control strategies of antibiotics and antibiotic resistance genes (ARGs) from wastewater of antibiotic manufacturing.
In this study, we have synthesized a detailed review of existing techniques and their integration for the removal of antibacterial potency from antibiotic production wastewater (Table 1). Physical processes, including coagulation, sedimentation, and adsorption using activated charcoal or alternative materials, as well as chemical oxidation methods, are characterized by low efficiency and selectivity for antibiotic removal. As a result, the expenses tied to these processes are relatively substantial. The hydrolysis of antibiotics can be enhanced by optimizing the pH, additionally heating the wastewater, or adding solid base catalysts (16–19). Employing semi-empirical prediction tools based on frontier molecular orbital (FMO) theory, correlations were established between the enhanced hydrolysis efficiency and the energy gap (ΔE) between the lowest unoccupied and highest occupied molecular orbitals (ELUMO and EHOMO). In this regard, enhanced hydrolysis is suggested for treating wastewater containing antibiotics such as tetracycline, oxytetracycline, penicillin V, erythromycin, spiramycin, streptomycin, chloramphenicol, vancomycin, bacitracin, and colistin (17). Enhanced hydrolysis demonstrates exceptional efficacy due to its selective destruction of antibiotic functional groups responsible for their potency. Notably, this approach has been successfully implemented in full-scale pharmaceutical wastewater treatment facilities in China (Figure 3) (20–24). The similar hydrolysis-based method has also been adapted for the treatment of solid waste from fermentative antibiotic production, which offers notable implications for the disposal of antibiotic fermentation residues (13,25). For wastewater that contains oil, employing yeast as a biological treatment strategy is favorable, as it prevents the emergence of bacterial antibiotic resistance (26-27). Moreover, advanced treatment strategies, such as synchronized oxidation-adsorption (SOA) (28), ozonation, and advanced oxidation processes (AOPs) using UV light with photosensitizers or Fenton’s reagent, should be considered as final safeguards before discharge. Membrane technology, such as reverse osmosis, particularly for water recycling, exhibits strong efficacy in mitigating antibacterial potency and ARGs in treated effluent, although it is cost-prohibitive (3). Presently, the optimized strategy for the treatment of antibiotic production wastewater involves a combination of biological treatment, pretreatment, and advanced treatment, with the goal of preventing the release of antibiotics and ARGs. This integrated method is strategically designed to comply with wastewater (Figure 2). Additionally, certain higher-cost techniques, such as mechanical vapor recompression (MVR), have been applied within the industry with satisfactory outcomes.
Available technologies Description Advantage and disadvantage Pretreatment techniques (16–19,26,27,32) Enhanced hydrolysis Pretreatment method used for production wastewater of fermentative antibiotics (for example, tetracyclines and macrolides). It can selectively remove antibiotics from wastewater by eliminating their active antibacterial groups. The removal of antibiotics could reach 99%, and the reduction of AMR discharge is about 80%. Selective hydrolysis of functional groups of antibiotics with low cost and decrease of inhibition on biological treatment and dissemination of ARGs in the environment. Biological technique using yeast Pretreatment method used for oil-containing antibiotic production wastewater. Oil residue removal rate was 61.4%–74.2% in full-scale operations, and oil is the substrate for the fermentation production of antibiotics. No ARG from bacteria produced since yeast play the role in the biological treatment. Avoiding the emergence of ARGs in bacteria during biological treatment. Coagulation, sedimentation and adsorption-based techniques Traditional pretreatment methods used for wastewater with high content of suspended solid. Some kinds of antibiotics can be partially removed, while the removal is very limited. Low removal of antibacterial potency. Oxidation-based techniques For example, ozone oxidation and Fenton oxidation. Doses of 1.2 mg O3 per mg of initial OTC permitted 92% OTC removal from OTC production wastewater (OTC, 702 mg/L). High cost and low selectivity for antibacterial potency removal. Advanced treatment (3,28) Oxidation-based techniques For example, SOA, ozone oxidation, Fenton oxidation, electrochemical oxidation. End protection before discharge. High cost. Membrane separation For example, ultrafiltration, reverse osmosis. Good removal of antibacterial potency and ARGs in effluent. High cost. Technique integration (5,20-24,33) Pretreatment +biological treatment The effluent needs to be discharged to a municipal wastewater treatment plant. With the pretreatment such as enhanced hydrolysis, the antibacterial potency could be removed. Pretreatment + biological treatment + advanced treatment It can meet the standards for direct emission. With the pretreatment such as enhanced hydrolysis, the antibacterial potency could be removed. MVR + harmlessness of solid waste and waste gas Maximizing the recovery of water from wastewater using vapor. No wastewater discharge. High cost. Disposal of solid waste is difficult. Abbreviation: ARGs=antibiotic resistance genes; AMR=antimicrobial resistance; OTC=oxytetracycline; SOA=synchronized oxidation-adsorption; MVR=mechanical vapor recompression. Table 1. Available techniques and technique integration for removing antibacterial potency from antibiotic production wastewater.
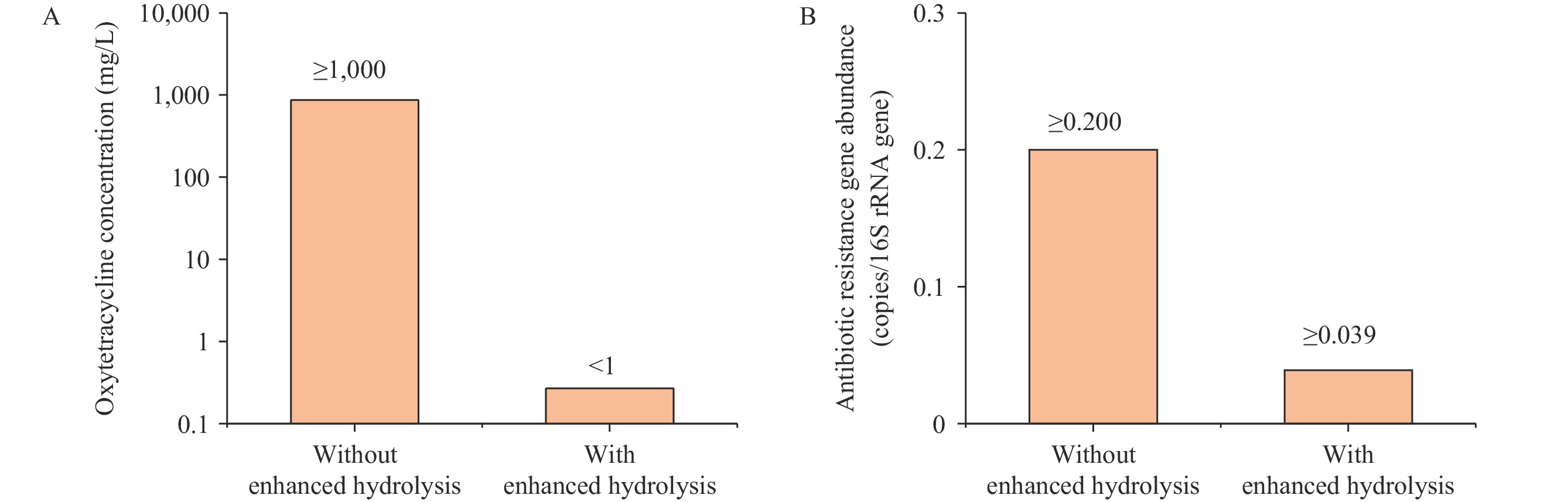 Figure 3.
Figure 3.Reduction of discharge of antibiotics (A) and antibiotic resistance genes (B) from biological oxytetracycline production wastewater treatment system with enhanced hydrolysis pretreatment.
Note: This figure was modified from Yi et al. (2017) (24). -
In China, numerous successful instances of AMR control within antibiotic production sectors provide instructive benchmarks for implementing global AMR containment strategies (29). From the standpoint of engineering applications, the efficacy of enhanced hydrolysis pretreatment has been convincingly established through a range of full-scale implementations by pharmaceutical corporations across the country. Importantly, this method has been incorporated into and recommended by the WHO’s technical brief, and has been endorsed by the standards of the Ministry of Ecology and Environment of China (Table 2). The China Pharmaceutical Enterprises Association (CPEA) has documented additional full-scale applications in its “Compilation of Excellent Cases in the Pharmaceutical Industry for Environment, Health, and Safety (EHS),” covering the period from 2019 to 2021.
Areas Description Source or impacts Engineering application Pretreatment (enhanced hydrolysis), biological treatment and advanced treatment (low-cost SOA technology) were successfully applied in full-scale pharmaceutical wastewater treatment plants in Hebei Province and Jiangsu Province in China. Pretreatment of antibiotic production wastewater to remove antibacterial potency is the best way to control the development of ARGs, as well as the above successful cases, has been subsumed in “Technical brief on water, sanitation, hygiene and wastewater management to prevent infections and reduce the spread of antimicrobial resistance” (2020) [ISBN (WHO) 978-92-4-000641-6] and Ministry of Ecology and Environment of China standard (HJ 1305-2023). Technique integration including the pretreatment (e.g., electrochemical oxidation, iron-carbon microelectrode, MVR), biological treatment, advanced treatment (e.g., ozone oxidation) or zero emission (e.g., MVR+ special membrane) were applied in some plants in China. Some cases were included in the Compilation of Excellent Cases Pharmaceutical Industry Environment, Health and Safety (EHS) (2019–2021) by CPEA (http://www.cpema.org/). Environmental management A chapter of “Synergistic control of antibiotics, resistance genes and conventional pollutants in pharmaceutical wastewater”. “Bluebook of pharmaceutical Industry” (ISBN 9787520170635) was published by CPIA in 2020. API management and PNECs in pharmaceutical wastewater. Pharmaceutical Industry Environment, Health and Safety (EHS) Guide (2020) by CPEA (http://www.cpema.org/). Three standards for determining erythromycin, cephalosporin and penicillin in antibiotic fermentation residue, raw fertilizer material, crop, and related environments. CPIA (TPIAC00001-2021, TPIAC00002-2021, and TPIAC00003-2021). A series of available techniques including the enhanced hydrolysis pretreatment for API removal were summarized in this standard, which is an important criterion on pollution (including antibiotics) control in pharmaceutical industry. Standard by Ministry of Ecology and Environment of China: “Guideline on available techniques of pollution prevention and control for pharmaceutical industry (fermentation, chemical synthesis, extraction) and preparation categories” (HJ 1305-2023). Abbreviation: CPEA=China Pharmaceutical Enterprises Association; CPIA=China Pharmaceutical Industry Association;SOA=synchronized oxidation-adsorption, MVR=mechanical vapor recompression, API=active pharmaceutical ingredient, PNECs=Predicted No-Effect Concentrations, ARGs=antibiotic resistance genes. Table 2. Some typical successful cases of AMR control from antibiotic production wastewater in antibiotic manufacturing in China.
In recent years, from an environmental management perspective, Chinese government agencies and relevant industry associations have invested considerable effort into the establishment of standards and the advancement of technology (Table 2). The China Pharmaceutical Industry Association (CPIA) published the “Blue Book of Pharmaceutical Industry” (ISBN 9787520170635) in 2020, which includes a chapter by the Research Center for Eco-Environmental Sciences, Chinese Academy of Sciences, on “Synergistic Control of Antibiotics, Resistance Genes, and Conventional Pollutants in Pharmaceutical Wastewater.” The “Pharmaceutical Industry EHS Guide (2020)” — issued by the CPEA — recommended API management strategies and related Predicted No-Effect Concentrations (PNECs).
Additionally, the CPIA released three standards (TPIAC00001-2021, TPIAC00002-2021, and TPIAC00003-2021) aimed at determining levels of erythromycin, cephalosporin, and penicillin in antibiotic fermentation residue, raw fertilizer materials, crops, and associated environments. This trio of standards marks China’s inaugural effort to standardize the determination of antibiotics in antibiotic fermentation residue. More significantly, the Ministry of Ecology and Environment of China introduced the “Guideline on Available Techniques of Pollution Prevention and Control for the Pharmaceutical Industry (Fermentation, Chemical Synthesis, Extraction) and Preparation Categories” (HJ 1305-2023). This authoritative guideline compiles a range of available techniques, such as enhanced hydrolysis pretreatment for API removal, and sophisticated end-protection treatments. It stands as a critical benchmark for regulating pollution, including antibiotics, within the pharmaceutical industry.
In 2023, at the PSCI China Supplier Conference in Chengdu, a poll was conducted to assess the implementation of AMR control measures by pharmaceutical companies. Out of 78 Chinese supplier representatives who participated, 42 (representing 54%) voted in favor. This outcome suggests that while the notion of mitigating AMR in antibiotic manufacturing is widely recognized, there remains a significant need for concrete action. It is apparent that enhancing awareness and promoting the dissemination of current progress and available technologies in the industry are crucial steps toward bolstering the fight against AMR.
Apart from China, India is also a leading exporter of antibiotics worldwide. The country is progressively implementing responsible manufacturing practices and placing greater demands on corporate compliance. All bulk-drug API manufacturing facilities are designated as “grossly polluting industries” and must comply with Zero Liquid Discharge standards (30). In 2019, the Indian government introduced legislation proposing discharge limits for antibiotics in wastewater emanating from production sites into surrounding water bodies. However, the definitive legislation published in 2021 ultimately did not incorporate these discharge limits (31).
Growing concerns about AMR in the pharmaceutical industry necessitate immediate, strategic actions. The development of an AMR management system tailored to Chinese conditions, along with increased research and advancement of risk control technologies, are imperative for fostering sustainable industry practices. Looking ahead, there are critical areas that require heightened focus and commitment.
First and foremost, concerted efforts should be directed towards the long-term risk identification, evaluation, and mitigation of AMR stemming from the disposal of antibiotic production wastewater and fermentation residues. Additionally, comprehensive guidelines and a strategic roadmap, encompassing systematic AMR management standards, must be crafted collaboratively by industry stakeholders and cross-sectoral entities. Furthermore, the industrial adoption of cost-effective and efficient methods represents a key solution for curbing the spread of AMR attributable to antibiotic manufacturing. Prioritizing the reduction of antibiotics and ARGs emissions from the pharmaceutical sector into the environment remains of paramount importance.
HTML
Characteristics of the AMR Crisis in the Pharmaceutical Industry
Trends in the Management of Antimicrobial Emissions from Antibiotic Production Facilities Globally
Techniques for Removing Antibacterial Potency from Antibiotic Production Wastewater
Management of AMR in Antibiotic Production in China
| Citation: |




 Download:
Download:




