-
Antimicrobial resistance (AMR) refers to microorganisms (i.e., bacteria, fungi, viruses, and parasites) that can become resistant to antimicrobials through a variety of mechanisms such as mutation or genetic exchange. The world is facing high rates of AMR — particularly antibiotic resistance — which threaten the core of modern medicine and the sustainability of an effective, global public health response to the enduring threat of infectious diseases. The response to the AMR crisis has been spearheaded through the One Health AMR Globe Action Plan (GAP) (1), which was developed by the World Health Organization (WHO) in close collaboration with the Food and Agriculture Organization of the United Nations (FAO) and the World Organization for Animal Health (WOAH). The One Health perspective emphasizes close connections among humans, animals, and the environment (2). It is frequently demonstrated that clinically important resistance genes or resistant bacteria could be disseminated among humans, animals, and the environment. For example, carbapenemase-producing Escherichia coli were reported to transmit among humans and backyard animals (3); tet(X)-variant genes were reported to disseminate from layer farms to manure-receiving soil and corresponding lettuce (4). The generation and spread of resistant bacteria and antibiotic resistance genes (ARGs) in the environment will have direct and immediate health implications for both humans and animals.
It has been recognized that the environment is the potential source, reservoir, and transmission route of AMR. Antibiotic resistant bacteria (ARB), antibiotics, and other selective agents will be discharged into the environment. ARGs can be shared between bacteria under selective pressure from antimicrobials along with other selective agents. The sharing allows for spread of AMR across diverse populations of environmental bacteria and pathogens. People and animals can be exposed to AMR through the intake of food, water, and air.
-
Natural AMR is common among environmental bacteria, including in pristine locations relatively untouched by anthropogenic activities (5-7). However, the use of antimicrobial agents in humans and animals has been associated with the evolution and amplification of antimicrobial resistant pathogens and their respective ARGs. Anthropogenic activities are increasing the importance of the environment as a pathway for human AMR exposure. Release of antimicrobials or other selective agents into the environment via excreta from humans, terrestrial or aquatic animals, or from antimicrobial manufacturing waste and wastewater promotes resistance by creating an environment that is favorable for the transfer or emergence of new resistance genes (8). There are three main emission sources in the environment: animal farming, hospital/community sewage, and the pharmaceutical industry (Figure 1).
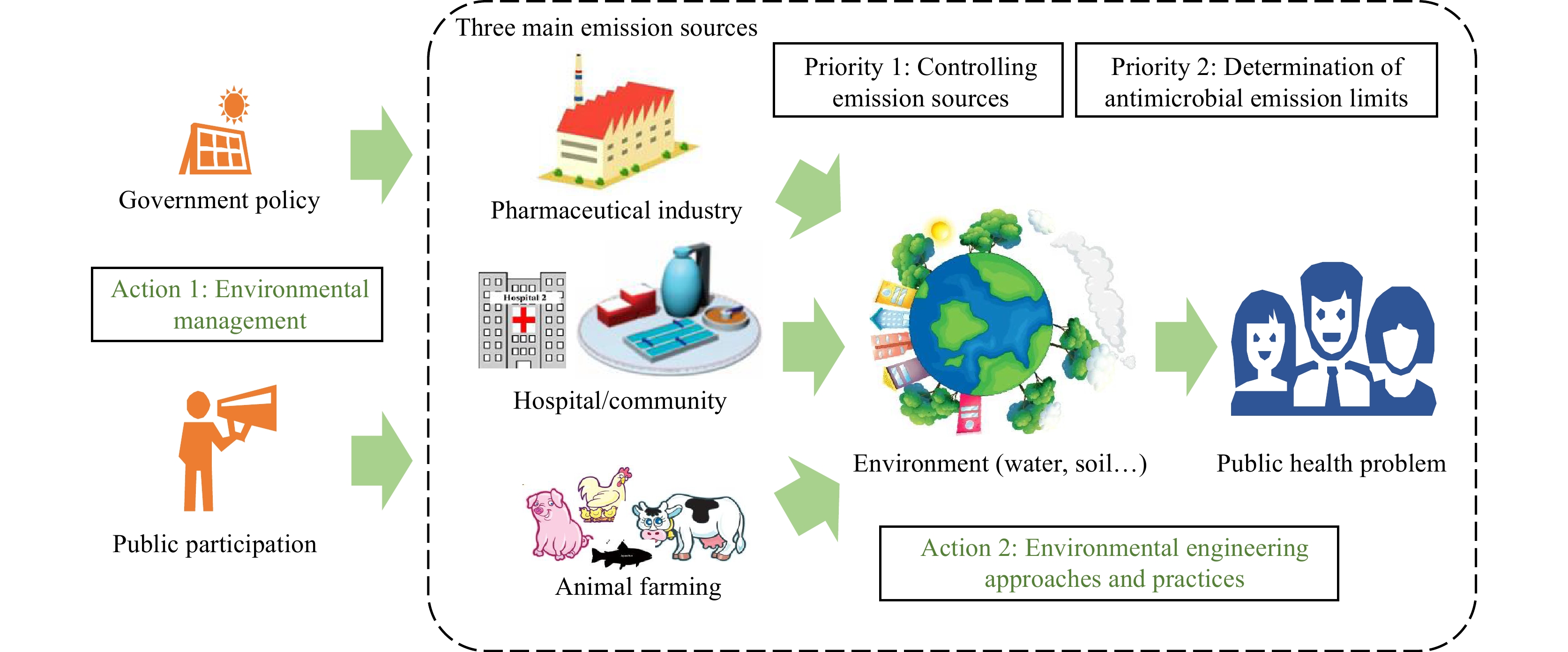 Figure 1.
Figure 1.Environmental mission sources, priorities, and actions for minimizing risk of developing AMR in the environment.
Abbreviation: AMR=antimicrobial resistance.Human consumption of antimicrobials can result in antimicrobial resistant pathogens and ARGs being discharged to the environment. Wastewater from hospitals, intensive livestock farms, and aquaculture is likely to contain particularly elevated concentrations of antimicrobials, ARB and ARGs, which might influence AMR spread (8). Wastewater discharge from antimicrobial production is a hotspot for AMR development. Antibiotics in water downstream of some antibiotic manufacturing sites have been found at higher concentrations than those found in the blood of patients taking medicines (9), facilitating AMR selection in the local environment. The conventional wastewater treatment processes are typically not designed for treating high concentrations of antimicrobials. Thus, the development of AMR during pharmaceutical wastewater treatment is a universal problem.
-
To contain AMR dissemination in the environment, the priority is to minimize the emission of antimicrobials and ARB/ARGs during the discharge of waste and wastewater from animal farming, hospitals, and pharmaceutical manufacturing (Figure 1). Since antimicrobials could become selective agents for AMR once released into the environment, the most important approach is to reduce the residual level in wastewater as much as possible. However, the reduction of antimicrobials has not yet been set as a target for wastewater treatment. Meanwhile, AMR development could also occur during wastewater treatment under the selective pressure of the antimicrobials. Thus, pre-treatment of production wastewater to remove antimicrobials is the best way to control the development of ARGs during biological wastewater treatment; this has been implemented in some manufacturing sites in China (8). It is also important to prevent the release of the ARB and ARGs in wastewater to the environment. Different disinfection technologies could be used for this purpose. On the other hand, rapidly developing membrane technology could provide an attractive option because, unlike disinfectants, it will impose no selective pressure on bacteria.
-
To minimize the emissions of antimicrobials, it is necessary to establish a sound emission limit (Figure 1). Currently, there are no global effluent water quality guidelines based on health risk assessment. Recently, a voluntary industry group named the AMR Industry Alliance has developed initiatives to establish a common manufacturing framework for managing the discharge of antimicrobial compounds into waterways and apply it across manufacturing and supply chains among their members. One of the important points in this framework is to determine predicted no-effect concentrations (PNECs) to mitigate against AMR spread (10). The PNECs for resistance selection were acquired by modeling based on the Minimal Inhibitory Concentrations of 111 antibiotics from the public European Committee for Antimicrobial Susceptibility Testing (EUCAST) database (11). The current list stands at 125 antibiotics (12). However, transforming such a list into internationally accepted emission targets is still a challenge. While this list only focuses on emission control, it is also important to establish a guideline for the pre-treatment of production wastewater.
-
Table 1 summarizes some important actions related to AMR containment in the environment. Many countries are taking actions to limit emissions. For example, China has included waste antibiotic fermentation residue in the national hazardous waste list, and the disposal and discharge of antibiotic residues are strictly regulated. Norway and Sweden have added emission control as part of their antimicrobial procurement criteria. WHO and other agencies have summarized recent progress of AMR control in a technical brief on antimicrobial resistance in 2020 (8). The list of antibiotic manufacturing discharge targets established by the AMR Industry Alliance was adopted by the Pharmaceutical Supply Chain Initiative (PSCI). A pharmaceutical industry Environment, Health, and Safety (EHS) guideline issued by the China Pharmaceutical Enterprises Association (CPEA) suggested monitoring the active pharmaceutical ingredients (APIs) in waste and wastewater, establishing an effective management system, and formulating emission control targets in accordance with PNECs. Pretreatment of antibiotic production wastewater to remove the residual antibacterial activity was included in the WHO technical brief (8) and the Blue Book of China’s Pharmaceutical Industry (2020), published by the China Pharmaceutical Industry Association (CPIA), as an approach to ensure the synergistic control of antibiotics, ARGs, and conventional pollutants (13).
Action types Year Actions International actions 2013 Green procurement for pharmaceutical manufacturing (WHO and UNEP meeting) 2015 Global Action Plan on AMR (WHO) 2016 Creation of the AMR Industry Alliance 2017 Developing priorities for WHO activities on anti-microbial resistance and the environment (WHO expert meeting) 2018 List of antibiotic manufacturing discharge targets following the launch of the Common Antibiotic Manufacturing Framework (AMR Industry Alliance) 2019 Antibiotic use and wastewater residue (WHO expert meeting) 2020 Technical brief on water, sanitation, hygiene and wastewater management to prevent infections and reduce the spread of antimicrobial resistance (WHO/FAO/WOAH) 2022 Antibiotic manufacturing standards: Minimizing risk of developing antibiotic resistance and aquatic ecotoxicity in the environment resulting from the manufacturing of human antibiotics (AMR Industry Alliance) Actions of China 2002 Antibiotic fermentation residues were listed as prohibited drugs in forage and animal drinking water 2008 Antibiotic fermentation residues were officially included in Directory of National Hazardous Wastes in 2008 and remained in the revised versions in 2016 and 2021 2016 National action plan to contain antimicrobial resistance in China (2016–2020) 2017 Ministry of Agriculture and Rural Affairs of China formally banned colistin as an animal growth promoter 2019 Ministry of Agriculture and Rural Affairs withdrew all types of growth promoters from animal feed except Chinese medicine on July 10, 2019, and the ban took effect on January 1, 2020 2020 Pharmaceutical Industry EHS Guide (CPEA) 2020 Annual Report on the Development of China's pharmaceutical Industry with a chapter on “Synergistic control of antibiotics, resistance genes and conventional pollutants in pharmaceutical wastewater” (CPIA) 2021 The Biosecurity Law of the People’s Republic of China was issued. Containment of AMR was emphasized 2021 Standards for determination of erythromycin, cephalosporin and penicillin in antibiotic fermentation residue, raw fertilizer material, crop, and related environments (CPIA) 2022 The National action plan to contain antimicrobial resistance in China (2022–2025) Abbreviation: WHO=World Health Organization; UNEP=United Nations Environment Programme; FAO=Food and Agriculture Organization of the United Nations; WOAH=World Organization for Animal Health; CPEA=China pharmaceutical Enterprises Association; EHS=Environment, Health and Safety; CPIA=China Pharmaceutical Industry Association. Table 1. Summary of some important international and Chinese actions related to AMR containment in the environment since 2002.
On the other hand, some actions from one health perspective have been adopted. Following the discovery of mcr-1 (14), the Ministry of Agriculture and Rural Affairs of China banned colistin as an animal growth promoter. The colistin withdrawal policy and the decreasing use of colistin in agriculture have had a significant effect on reducing colistin resistance in both animals and humans in China (15). China has decided to ban antibiotics as growth promoters in animal feed. However, there are no internationally agreed actions on the containment of the emissions of antimicrobials and ARB/ARGs to the environment. It is desirable to establish a holistic framework to coordinate international actions on the containment of environmental AMR development. Recently, China has announced a new national action plan (NAP 2022–2025, Table 1) to combat antimicrobial resistance. The plan embraces the one-health concept, with health, agriculture and environmental protection departments dedicated. It also details measures to contain the dissemination of antimicrobial resistance in the environment, such as enhancing the control of antimicrobial pollution discharge and carrying out trials to monitor antimicrobials in water environments. In addition, cross-resistance of antibiotics used in animals and humans becomes an increasingly serious problem. More actions on reasonable arrangement of prophylactic and therapeutic veterinary antibiotics from the one health perspective are needed.
-
The biological processes — a combination of anaerobic digestion and an activated sludge process — widely used in wastewater treatment are vulnerable to the presence of extremely high concentrations of antibiotics in antibiotic production wastewater. The presence of high concentrations of antibiotics in wastewater has been found to cause treatment failure by disturbing wastewater treatment microbial communities. Particularly, multidrug resistance can be developed during wastewater treatment due to horizontal gene transfer of ARGs among the bacterial community mainly through the enrichment of plasmids harboring multidrug resistance regions (16–17). Motivated by the ease of hydrolysis of most antibiotics, a novel pretreatment technology based on enhanced hydrolysis has been developed using homogeneous or heterogeneous acid/base catalysts for targeted elimination of antibiotic potencies from wastewater (18–19). The enhanced hydrolysis pre-treatment technology has been successfully applied in full-scale plants in Hebei Province, China (13). The antibiotic concentrations could be reduced from around 1,000 mg/L to less than 1 mg/L through the pre-treatment. The abundance of ARGs in the biological treatment units could be reduced by >80%, and the biological wastewater treatment systems could be operated under stable conditions. In addition, hydrothermal treatment based on enhanced hydrolysis was also applied in Chinese full-scale plants for recycling waste erythromycin and cephalosporin fermentation residues (20).
Similarly, livestock waste is another major source of ARGs in the environment. Some prevalent high-risk ARGs in animal manure, such as CTX-M-type extended spectrum
$\beta$ -lactamase genes (blaCTX-M), the ABC transporter gene conferring resistance to florfenicol and linezolid (optrA), and mobile tigecycline resistance gene [tet(X) variants], were found to persist in animal manure, mesophilic anaerobic digestion and composting systems, and the related environment (21-23). Hyperthermophilic anaerobic treatment or composting could reduce the abundance of most high-risk ARGs and inactivate the fecal bacteria effectively, indicating that effective management of operating temperatures in anaerobic digestion or composting might be an effective way to prevent the discharge of the high-risk ARGs from animal manure treatment systems.The role of the environment in the rise, spread, and health risks of AMR have been intensively investigated. However, environmental AMR development is an extremely complicated issue. There are still many questions to be answered: How and to what extent do different sources of antimicrobial residues and ARB contribute to developing AMR in the environment? How can an internationally coordinated action plan be established to contain environmental AMR development? To better answer these questions, coordinated monitoring, research, and actions are required. In 2016 and 2022, China developed its own National Action Plan with contributions from multisectoral departments for 2016–2020 and 2022–2025, respectively (Table 1). China is advocating establishing a community with a shared future for humanity; therefore, an important role in such critical international cooperation to cope with AMR challenges could and should be played.
-
Ziming Han and Shihai Liu for visualization and formatting.
-
No conflicts of interest.
HTML
Minimizing Emissions
Determining Antimicrobial Emission Limits
Environmental Management
Environmental Engineering Approaches and Practices
| Citation: |



 Download:
Download:





