-
On August 20, 2021, a human plague case (55-year-old woman) suffered bubonic plague with secondary septicemic plague was reported from General Hospital of Ningxia Medical University (GHNMU) in Ningxia Hui Autonomous Region (Ningxia), China. Effective patient’s treatment, together with enhanced rodents plague surveillance and control, was performed in Ningxia and Inner Mongolia Autonomous Region (Inner Mongolia). The genome-wide single nucleotide polymorphism (SNP) analysis was used in source tracing based on phylogenetic relationship of Yersinia pestis (Y. pestis) strains in this event. The Y. pestis strain isolated from the patient in Ningxia and the strain from local Meriones unguiculatus (M. unguiculatus) that were found near the patients’ residences were clustered into the same lineage (2.MED3q). Such observations indicated that human plague cases originated from local reservoirs.
-
On August 20, 2021, a human plague case suffering from bubonic plague with secondary septicemic plague was reported from GHNMU in Ningxia. The patient was a 55-year-old female herdsman who lived in Wulan Village, Otog Qi (County), Erdos City in Inner Mongolia. On August 14 and 15, 2021, the patient presented with the onset of nausea and vomiting with low blood pressure and sought treatment in a village clinic but the patient’s condition deteriorated further. On August 16, 2021, the patient was admitted to the local county hospital in Pingluo County of Ningxia for high fever (40 °C) with weakness and vomiting. Subsequently, on August 17 later, the patient was transferred to GHNMU in Yinchuan City and was admitted to the intensive care unit (ICU) for septic shock symptoms with left inguinal lymphadenitis. During this period, no coughing, chest pain, or breathlessness was observed. Thereafter, the blood of patient was conducted a bacteria culture and examined through biochemical analyzer in GHNMU, and the results of biochemical analyzer reported that bacteria in the patient’s blood was Yersina genus. Such results led the clinical doctors in GHNMU began to suspect the patient might suffer from plague and reported to the Ningxia CDC on August 20.
This human plague case was confirmed via polymerase chain reaction (PCR) positive results in lymph node aspirates and blood by the Ningxia CDC within four hours, targeting the caf1, pla, and YPO0392 genes of Y. pestis (1), as well as the positive results of the colloidal gold-immunochromatography assay and reverse indirect hemagglutination assay (RIHA) test targeting the F1 antigen in lymph node aspirates and blood. The titer of RIHA for the lymph node aspirates and blood were 1∶64 and 1∶128, respectively. In addition, the bacteria isolated from blood were identified as Y. pestis by Gram staining, microscopy, and a phage lysis test. While, the PCR assays for the patient were negative in the sputum and throat specimens of the patient.
Based on clinical manifestations and laboratory test results, the patient was diagnosed with bubonic plague with secondary septicemic plague and she was treated with antibiotics (streptomycin and ciprofloxacin) and recovered on September 6, 2021.
One Y. pestis (Ningxia 2021) isolated from the 55-year-old patient was sequenced by Ningxia CDC. Meanwhile, one strain (Neimeng 2021) isolated from M. unguiculatus eighty meters away from the house of the patient was isolated and sequenced by Inner Mongolia CDC. Under the source tracing mechanism of the Chinese Pathogen Identification Net (CPIN), the patient-related (Ningxia 2021), the strain from M. unguiculatus in Inner Mongolia (Neimeng 2021), Y. pestis strains isolated from Inner Mongolia in 2019, together with Y. pestis genomes in CPIN were compared by the genome-wide SNPs (2). As shown in Figure 1A, the strain isolated from the patient and the strain isolated from M.unguiculatus 80 meters away from the houses of patient were clustered as 2.MED3q lineage, a lineage inherently belonging to the Y. pestis in the Erdos Plateaus M. unguiculatus plague focus in Inner Mongolia in 2019 (2).
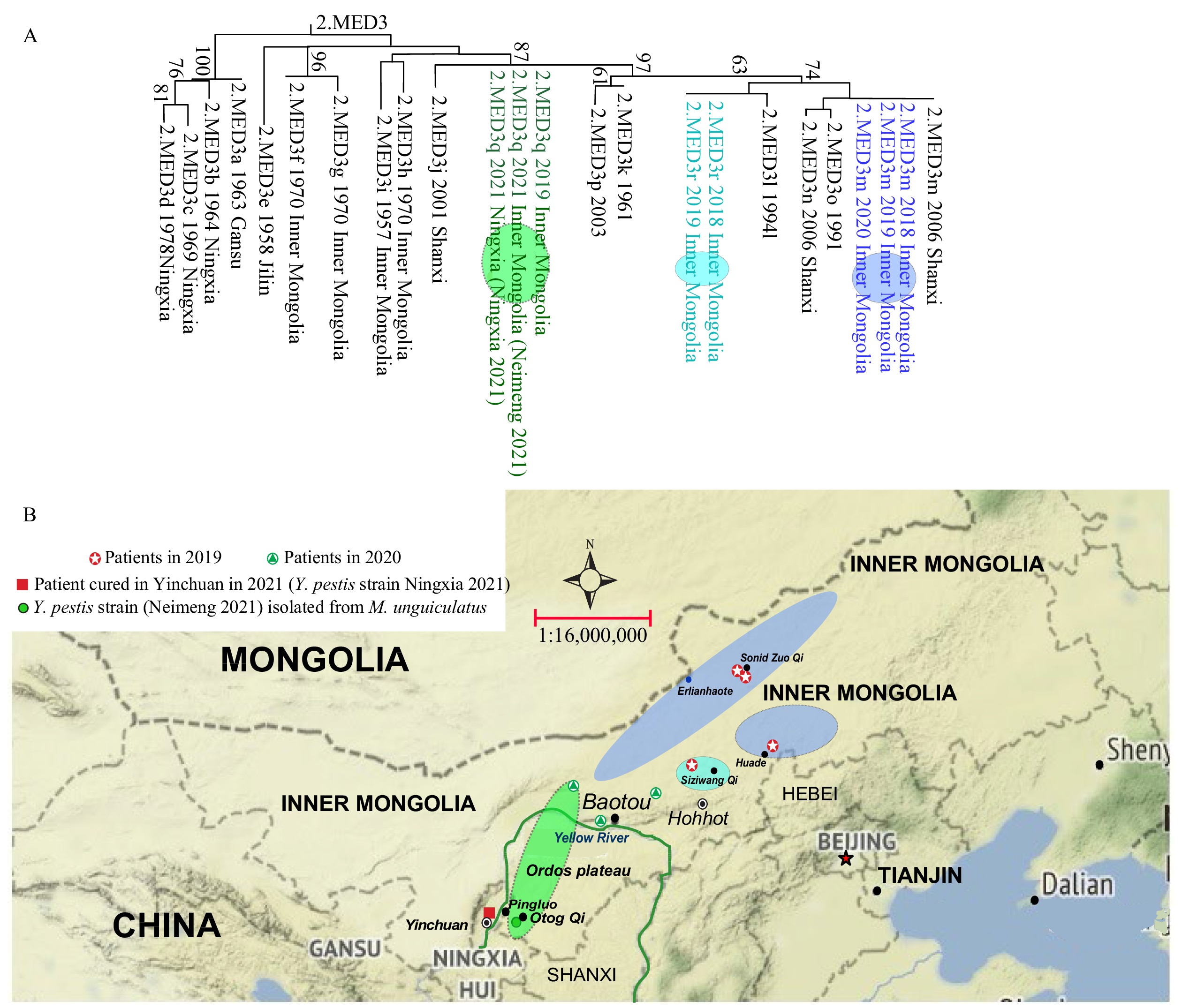 Figure 1.
Figure 1.Geographical distribution and phylogenetic relationship of patients and various epizootics in Inner Mongolia and Ningxia. (A) Phylogenetic relationship based on whole genomic SNPs in Y. pestis 2. MED3 population. (B) Geographical distribution of phylogenetic lineages in Inner Mongolia.
Note: The maximum likelihood tree in Figure 1A was constructed by the concatenated SNP using MEGA (Molecular Evolutionary Genetics Analysis Version 6.0. Tamura K, Stecher G, Peterson D, Filipski A, and Kumar S, 2013). Strains in colored circle on evolutionary trees isolated from the sites with same color on the map.In this Y. pestis infection event, the patient was a shepherd, and the family of the patient lived in a comparatively independent house that was located on the Ordos Plateaus M. unguiculatus plague focus in Inner Mongolia. There were a total of six families together with the patient’s family within a three-kilometer radius away from the house of the patient. According to reports from local residents, in the past two months, several dead M. unguiculatus had been found in the region before the case of human plague occurred. Because there were a total of four and three human plague cases occurred in the Inner Mongolia in 2019 and 2020, respectively, with 1 and 2 deaths in 2019 and 2020, respectively, and there were 2 Y. pestis strains isolated from M. unguiculatus plague focus in Otog Qi in 2019, thus regulations were performing in Inner Mongolia that the local residents should be alerted to dead reservoirs and were required to report to local CDCs once the affected areas were found. The family of patient just had moved from Ningxia and been employed by a local inhabitant for only one year, so they did not know of the regulations and neglected to report dead rat phenomenon. Other five families’ residents reported the abnormal dead rats phenomenon, and local professional staff conducted rodenticides in residential surrounding areas, but nothing less than the areas patient lived in was left.
After the human plague was confirmed, an enhanced rodent surveillance campaign was performed. A total of 2 recently dead M. unguiculatus were found, and the Y. pestis strain was isolated from each dead rat, with one just 80 meters away from the house of patient; in addition, over 70 fleas were found on the rat. In addition, there were a large number of fleas inhabiting those reported reservoirs. Such an observation indicated that serious M. unguiculatus plague epizootics with plenty of fleas existed in the patient’s living areas and that the patient might have been infected from a flea bite.
-
First, before the 55-year-old patient was diagnosed as having plague, she sought treatment in 4 clinics or hospitals in Inner Mongolia and Ningxia. Therefore, it is critical to strengthen professional training for local clinicians in order to recognize and identify the disease earlier and be alerting to various forms of plague. In addition, the patient suffered from bubonic plague with secondary septicemic plague; unlike the pneumonic plague, the bubonic and the septicemic plague has limited the ability of person-to-person transmission (3). Therefore, more reasonable public health measures should be recommended in affected areas.
Secondly, since the human plague cases occurred in 2019 and 2020, the local CDCs had offered the local farmers or shepherds a Health Box [which included insect repellent (DEET), thermometer and report card et al.]. Corresponding educational efforts should be enhanced to promote behaviors such as wearing long pants and applying DEET to anyone engaged in outdoor activities in plague focus area; reporting rodent die-offs; avoiding direct contact with sick or dead wild animals (e.g., foxes or rabbits); using insecticides and rodenticides to eliminate the fleas and the host animals simultaneously, or insecticide be priority to animal plague control.
Thirdly, the Ordos Plateaus M. unguiculatus plague focus in Inner Mongolia is also adjacent to Ningxia and Shanxi provincial-level administrative divisions (PLADs) in China (Figure 1B). There are no obvious geographic barriers between the M. unguiculatus plague focus in Inner Mongolia and its counterpart area in adjacent PLADs, so the joint prevention and control, including early joint warning and risk communication are necessary.
In addition, in the process of responding to the human plague events in China, the clinics commonly responsible for finding and treating patients, while the various level CDCs confirmed the diagnosis according to evidence based on laboratory assays. In this event, the biochemical analyzer in GHNMU reported that bacteria in the patient’s blood were of the Yersinia genus, even though it did not definitively that the sample was Y. pestis (due to lacking a corresponding database of bacteria), the clinicians began to suspect that the patient might have plague. It was the second time that such situation had occurred in China as the first occurred in Yunnan Province in 2016 (1). In fact, with the gains in technical competence in hospitals, there are many techniques such as the genome sequencing, biochemical analyzers, and mass-spectrometric techniques, that can firstly give the clinicians the clues to plague.
-
The M. unguiculatus plague focus in Inner Mongolia can be divided into two parts: the Ordos Plateaus and the desert steppe of the Ulanqab plateaus. Previous studies provided insight into the relationship between plague intensity and the level of precipitation in the semi-arid grasslands of Inner Mongolia (4). i.e., Plague epizootics depend on changes in the density and distribution of local M. unguiculatus (3), while elevated rainfall facilitates increasing population levels of M. unguiculatus (4). A great increase in the local rodent population was an obvious feature in Inner Mongolia M. unguiculatus plague focus in 2019 (2). In 2019, a comparatively higher density (3.1/hm2) of the major host in this focus than the historical average level (2/hm2) on the Inner Mongolia Plateau (5) was observed. In 2021, drought was the main feature in Ordos Plateaus. The density of the main host reached to 4.46/hm2, and high flea density is a major feature of Otog Qi in August, such as the percentage of host infested flea was 52.63% with host-flea index was 3.93 in Otog Qi in August 2021. Similar situations could be observed in the adjacent Pingluo county, the percentage index and host-flea index were 59.26% and 2.15, respectively, in August (local plague surveillance data). Such ecological factors, i.e., higher flea density, also aroused a more potent animal plague epidemic with more risk of human infection.
-
Plague is primarily a disease of wild rodents. Animal-to-animal transmission is mediated by flea bites, while human infection is often an accidental event, including being infected by the bites of escaped bacterium-bearing fleas. Continuous animal plague had occurred in previous years (in 2018, 2019, and 2020) in the M. unguiculatus Plague Focus in Inner Mongolia, and corresponding surveillance results indicated that animal plague epizootics were still active in the M. unguiculatus Plague Focus in Inner Mongolia (National Plague Surveillance report).
The Y. pestis strains inhabiting Inner Mongolia belong to 2.MED3 population. While Y. pestis strains in 2.MED3 population were further divided into various lineages (2.MED3a–p) in Inner Mongolia or adjacent PLADs such as Shanxi, Hebei, and Ningxia in China (6) (Figure 1). The Y. pestis strains isolated in 2018 and 2019 in Inner Mongolia could be divided into 3 lineages (2.MED3m, 2.MED3q, 2.MED3r) (2). Previous research found lineage 2.MED3m was a major lineage generally and affected most of the geographical area in Inner Mongolia M. unguiculatus plague focus in 2018 and 2019 (2). However, the patient-related stain was 2.MED3q lineage, which was mainly located in the Ordos Plateaus M. unguiculatus Plague Focus in Inner Mongolia (2).
-
No conflicts of interest.
-
Mu Guo from Yunnan Institute for Endemic Disease Control and Prevention; Colleagues from China CDC, Ningxia CDC, and Inner Mongolia CDC.
HTML
| Citation: |



 Download:
Download:




