-
Scrub typhus (ST) is a vector-borne rickettsial zoonosis caused by the organism Orientia tsutsugamushi, transmitted to humans by the bite of the larva of trombiculid mites (1-2). Up to the end of 2016 in China, all 31 provincial-level administrative divisions (PLADs) had recorded human cases, with the incidence increased by over 16-fold compared to that of 2006, with dramatic geographic expansions in both rural and urban areas and diversified seasonal patterns (3). Whether these profound epidemiological features might be related to clinical aspects remains obscure. This study described the epidemiological features and clinical outcomes of ST patients and assessed early predictors of severe disease by retrospective medical record review of 4,501 ST patients in 69 hospitals located throughout all 11 districts of Guangzhou City of southern China from January 2012 to December 2018. Severe ST was found to be associated with the decreased levels of albumin (ALB) and platelet (PLT) count and increased levels of serum creatinine (CREA) and total bilirubin (TBIL) in the blood, as well as the occurrence of dyspnea for ST patients, with estimated relative contributions more than 10% in the final boosted regression trees models, which could be helpful for the recognization and treatment of severe ST in the early clinical management.
According to the National Scrub Typhus Control and Prevention Guideline (2009) issued by China CDC, a total of 4,501 patients with clinically diagnosed and laboratory-confirmed ST were included in the study (Supplementary Figure S1). Demographic information, medical history, and exposure history were obtained by interviewing patients or their guardians. Clinical data which comprised of date of disease onset (the day when clinical signs or symptoms were noticed), signs and symptoms, laboratory measurements, imaging findings, and treatment regimens, were retrieved from medical records and collected by Epidata software (version 3.1; The EpiData Association; Odense, Denmark).
Patients who had ever developed any severe complications [multiple organ dysfunction syndromes (MODS), shock, or requiring intensive care unit (ICU) admission] during the hospitalization were defined as severe cases, and the remaining patients were defined as mild cases.
Continuous variables were summarized as medians and interquartile-range (IQR). Categorical variables were summarized as frequencies and proportions. Chi-squared test, Fisher’s exact test, or nonparametric test, were used as appropriate to determine the difference between groups. Multivariate logistic regression analysis and boosted regression trees (BRT) model were performed to examine the effect of clinical manifestations and laboratory indicators on illness severity and to attain an early prediction of severe ST. The area under the receiver operating characteristic curve (AUC) was calculated to evaluate the predictive power of the BRT model. The details on variable selection and the modeling analyses of multivariate logistic regression and BRT model were shown in the Supplementary Material and Supplementary Figure S2. A 2-sided P value of <0.05 is considered as statistically significant. All statistical analyses were performed using R software (version 3.6.2; R Foundation; Vienna, Austria).
A total of 5,354 hospitalized patients with ST from 69 hospitals were included for medical record screening, from which 115 patients had incomplete information, 17 had other infectious diseases, and 721 had vague diagnosis were excluded, which resulted in 4,501 patients with clinically diagnosed ST being included in the final analysis (Supplementary Figure S1), accounting for 76.1% of the total number of cases in Guangzhou City reported to the national surveillance system of China CDC (Figure 1A). The median (IQR) age of the patients was 57 (46–65) years, which gradually increased from 54 (42–62) in 2012 to 59 (49–66) in 2018 (P<0.001). A slightly higher proportion of female patients (54.4%) was observed, but no significant difference was shown across the study years (P=0.882) (Table 1). Overall, 72.6% of the patients resided in rural areas, with the proportion increased from 60.4% in 2012 to 80.0% in 2018, and farmers and retirees were the main occupations (Table 1). When geographically displayed, cropland and forest regions were related to higher case incidence, such as in Conghua, Zengcheng, Huadu, and Nansha districts (Figure 1B). Approximately 88.4% of the patients occurred between May and October. The median time from symptom onset to hospital admission was 7 (IQR: 5–9) days and the median length of hospital stay was 7 (IQR: 6–10) days. Almost all patients (93.1%) had received antibiotics treatment (including doxycycline, azithromycin, chloramphenicol, or levofloxacin), in combination with the supportive therapy (1.5%).
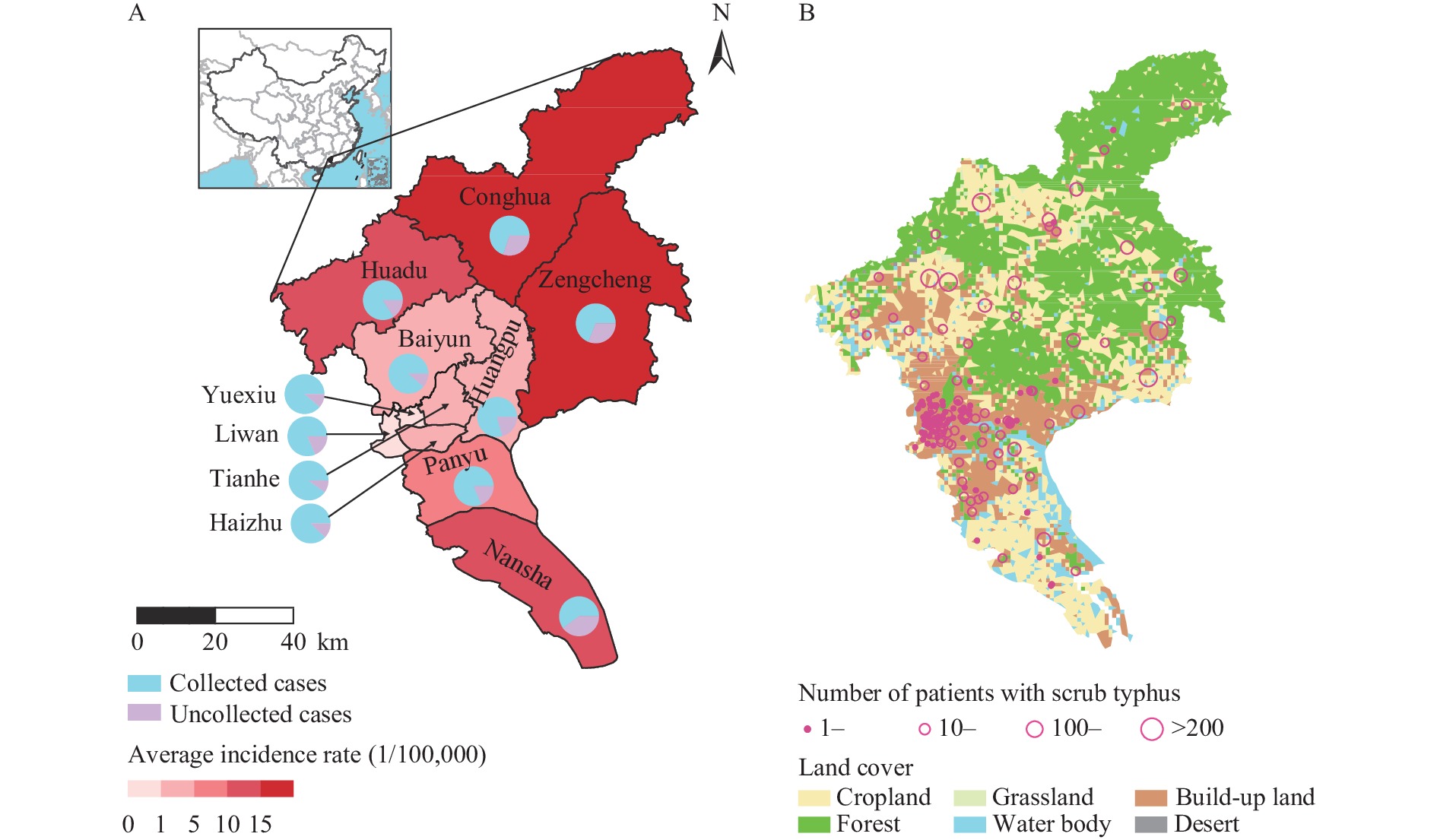 Figure 1.
Figure 1.The spatial distribution of scrub typhus in Guangzhou, China, 2012–2018. (A) The percentage of currently studied cases among all reported cases in each district of Guangzhou City according to National Surveillance Data, 2012–2018; (B) The overall number of reported patients by National Surveillance Systems of Infectious Diseases for each township, 2012–2018.
Variables Total
(n=4,501)2012
(n=558)2013
(n=673)2014
(n=731)2015
(n=606)2016
(n=617)2017
(n=610)2018
(n=706)P value* Sex, n (%) 0.882† Female 2,450 (54.4) 310 (55.6) 371 (55.1) 394 (53.9) 323 (53.3) 348 (56.4) 323 (53.0) 381 (54.0) Male 2,051 (45.6) 248 (44.4) 302 (44.9) 337 (46.1) 283 (46.7) 269 (43.6) 287 (47.0) 325 (46.0) Age, years, median (IQR) 57 (46–65) 54 (42–62) 56 (42–63) 56 (45–64) 58 (48–64) 58 (47–65) 59 (49–66) 59 (49–66) <0.001 Age group, years§, n (%) <0.001† 0–14 209 (4.6) 50 (9.0) 39 (5.8) 35 (4.8) 21 (3.5) 24 (3.9) 16 (2.6) 24 (3.4) 15–59 2,402 (53.4) 327 (58.6) 376 (55.9) 412 (56.4) 329 (54.3) 315 (51.0) 301 (49.3) 342 (48.4) ≥60 1,890 (42.0) 181 (32.4) 258 (38.3) 284 (38.8) 256 (42.2) 278 (45.1) 293 (48.0) 340 (48.2) Residence, n (%) <0.001† Urban 1,232 (27.4) 221 (39.6) 233 (34.6) 197 (26.9) 163 (26.9) 148 (24.0) 129 (21.1) 141 (20.0) Rural 3,269 (72.6) 337 (60.4) 440 (65.4) 534 (73.1) 443 (73.1) 469 (76.0) 481 (78.9) 565 (80.0) Occupation§, n (%) <0.001† Farmers 1,093 (24.3) 151 (27.1) 149 (22.1) 150 (20.5) 123 (20.3) 165 (26.7) 170 (27.9) 185 (26.2) Retirees 1,242 (27.6) 134 (24.0) 191 (28.4) 216 (29.5) 183 (30.2) 172 (27.9) 155 (25.4) 191 (27.1) Children and students 240 (5.3) 52 (9.3) 47 (7.0) 42 (5.7) 24 (4.0) 31 (5.0) 20 (3.3) 24 (3.4) Others 848 (18.8) 107 (19.2) 141 (21.0) 136 (18.6) 93 (15.3) 114 (18.5) 128 (21.0) 129 (18.3) Unknown 1,078 (24.0) 114 (20.4) 145 (21.5) 187 (25.6) 183 (30.2) 135 (21.9) 137 (22.5) 177 (25.1) Time from symptom onset to hospital admission, days, median (IQR) 7 (5–9) 7 (5–10) 7 (5–10) 7 (5–9) 7 (5–9) 7 (4–9) 7 (4–9) 6 (4–9) <0.001 Length of hospital stay, days, median (IQR) 7 (6–10) 8 (5–10) 8 (6–10) 8 (6–10) 7 (5–10) 7 (6–10) 7 (5–10) 7 (6–9) 0.092 Severe cases, n (%) 366 (8.1) 46 (8.2) 51 (7.6) 70 (9.6) 47 (7.8) 50 (8.1) 39 (6.4) 63 (8.9) 0.481† Death cases, n (%) 53 (1.2) 8 (1.4) 4 (0.6) 18 (2.5) 6 (1.0) 5 (0.8) 3 (0.5) 9 (1.3) 0.013† * P value relates the difference among the study years.
† P value calculated by use of χ2 test.
§ Some columns do not add up to 100% because of rounding.
Abbreviation: IQR=interquartile-range.Table 1. Baseline demographic and clinical characteristics of patients with scrub typhus in Guangzhou, China, 2012–2018.
Severe illness was determined from 366 patients, with a disease severity rate (DSR) of 8.1% (95% CI: 7.3%–8.9%), which did not differ across the studied years (Table 1, Figure 2A). Overall, 53 of the severe cases died, with a case fatality rate of 1.2% (95% CI: 0.9%–1.5%), which differed during the study years (Table 1). Patients with severe disease comprised 265 (5.9%) who developed MODS, 196 (4.4%) who were admitted to the ICU, and 139 (3.1%) who developed shock. When looking closely, children aged ≤14 years had a DSR of 11.0%, higher than that of the next age group of 15‒29 years old (2.0%); thereafter, an age-dependent increase of DSR was observed with patients ≥80 years having an 8-fold risk of DSR compared with the 15–29 years age group (Figure 2B). The DSR was comparable between males and females as a whole (8.2% vs. 8.1%, P=0.894). The median (IQR) time from symptom onset to hospital admission among severe patients was 8 days (6–10 days), significantly longer than that among mild patients (7 days, 4–9 days, P<0.001).
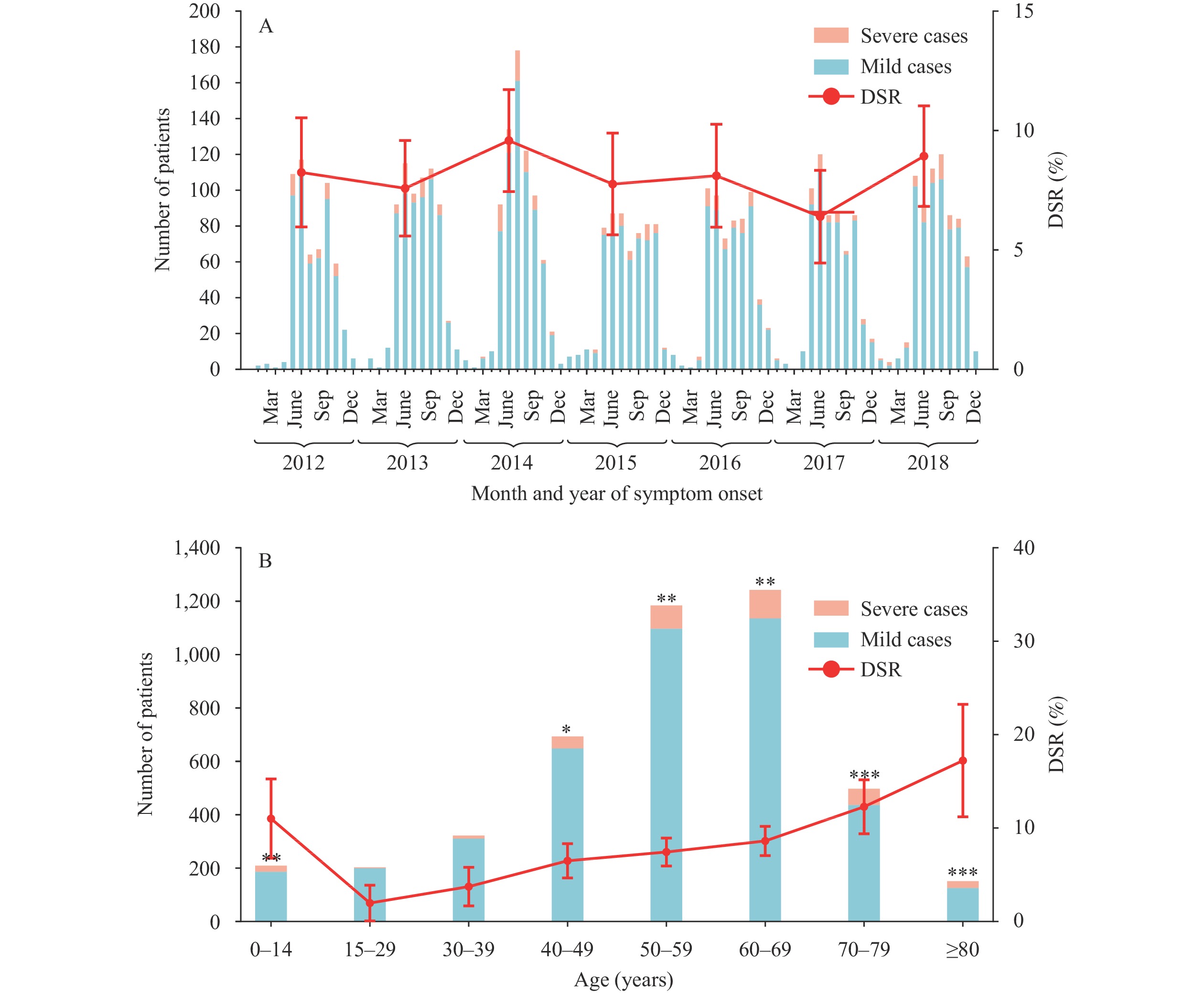 Figure 2.
Figure 2.Case number and disease severity rate of patients with scrub typhus by time and age in Guangzhou, China, 2012–2018. (A) Number of scrub typhus patients and annual disease severity rate (DSR) by month and year of symptom onset. (B) Number of scrub typhus patients and DSR of patients by age groups. P-value indicated association between age groups and severe disease by univariate logistic regression analysis, with 15–29 years group used as reference.
*, P<0.05; **, P<0.01; ***, P<0.001.The frequently seen symptoms and signs of ST patients included fever (98.2%), eschar (74.1%), anorexia (69.9%), headache (51.1%), weakness (43.7%), and cough (41.3%) (Supplementary Table S1). Ulcer and skin rash were less frequently seen (among 17.4% and 14.5% of the patients). By multivariate logistic regression analysis, 12 clinical manifestations were significantly related to severe disease, among which dyspnea had the most robust effect (adjusted OR: 13.95, 95% CI: 9.94%–19.58%), followed by confusion (OR: 7.18, 95% CI: 3.29%–15.67%), dysphoria (OR: 6.76, 95% CI: 2.40%–19.10%), lethargy (OR: 5.80, 95% CI: 2.19%–15.41%), macroscopic hematuria (OR: 5.32, 95% CI: 1.03%–27.39%), and edema (OR: 4.85, 95% CI: 3.25%–7.24%) (Supplementary Table S1).
A total of 28 laboratory parameters (12 hematological and 16 biochemical) were tested on admission, among which the frequently seen abnormalities included increased levels of neutrophil (NEU) percent in the blood, and aspartate aminotransferase (AST), alanine aminotransferase (ALT), lactate dehydrogenase (LDH), γ-glutamyl transpeptidase (GGT), and C-creative protein (CRP) in the serum above the normal range, decreased levels of hematocrit (HCT) in the blood, and total protein (TP), ALB, sodium (Na), and calcium (Ca) in the serum below the normal range (Supplementary Table S2). Multivariate logistic regression analysis disclosed laboratory abnormalities of PLT count, ALB, CREA, TBIL, hemoglobin (HGB), and NEU count that were significantly related to severe ST (Supplementary Table S3).
The final BRT model that included 10 factors attained a high prediction efficiency, with the mean AUC of 0.92 (95% CI: 0.90–0.93) and an accuracy of 0.88 (95% CI: 0.86–0.89) on testing data. The highest relative contribution (RC) to severe ST was observed for ALB, estimated to be 24.77% (95% CI: 23.70–25.85), followed by dyspnea, PLT count, CREA, and TBIL with their RCs more than 10% (Supplementary Table S4).
-
To our knowledge, this study represents the largest case-cohort study on ST patients. Notably, this cohort also represented properly treated patients by antibiotics, which had a high proportion of mild disease than those studies also including untreated patients in other Asian countries (4–5). The currently determined frequency of severe complications (8.1%) was lower than that of previous cohorts (6–7), although we had exhaustively included all complications, such as MODS, shock, and ICU admission, which are the principal causes of death in patients with severe disease. Even when properly treated, the 0–14 years pediatric patients were related to a higher DSR than its next age group of 15–29 years old. Whether this discrepancy was related to the higher incidence of contact to mites or due to the lack of herd immunity in children remained to be determined. More health education on ST among children should be implemented from schools and families in endemic areas at high risk of Orientia tsutsugamushi infection.
Uncommonly seen clinical complications, such as neurological manifestations, were present in 2.8% of the patients, which was based on an exhaustive search of the medical records, and were higher than those of a previous study in the Republic of Korea (1.7%) (8), while lower than studies in India and Thailand showing that up to 26% of ST patients had meningitis (9). Orientia tsutsugamushi has been suggested as an important cause of central nervous system infections in untreated patients. By contrast, among the properly treated patients with ST, the frequency of neurological can be significantly reduced, as displayed in the current study. The pathophysiological hallmark of ST is disseminated vasculitis, with subsequent vascular injury involved in skin, liver, brain, kidneys, and lungs, etc. Endothelial permeability may increase systemically and lead to the pathologic capillary leak syndrome resulting in tissue edema and intravascular hypovolemia (10). This can explain the prominent role of edema, as well as the increased laboratory abnormalities indicative of kidney and liver injury, i.e., CREA and TBIL. Decreased levels of plasma ALB and PLT count, surrogates of capillary permeability, were associated with severe disease.
Preexisting comorbidities significantly enhanced the odds of developing severe disease, with cerebral infarction showing significant effects according to the multivariate analysis. With no currently approved vaccines, aggressive treatment strategies should be applied and the treatment of a broad range of comorbidities should be advocated in those patients.
This study is subject to several limitations. Only hospitalized patients with ST were included for analysis, hence outpatients who were not admitted to hospitals warranted further investigation. Not all studied patients were laboratory-confirmed and misdiagnosis could not be completely ruled out, which could introduce uncertainty about our results.
In summary, we found that epidemiological and clinical features of ST were changing, with an increasing proportion of ST patients in the age ≥60 and in rural and higher DSR in children. Based on this knowledge, persistent surveillance and community health education should be stressed in high-risk populations, while severe ST patients could be recognized and treated in early clinical management.
-
No conflicts of interest declared.
HTML
| Citation: |



 Download:
Download:




