-
Although 23-valent pneumococcal polysaccharide vaccine (PPSV23) is recommended for patients with chronic obstructive pulmonary disease (COPD) to prevent acute exacerbations (AECOPD) from pneumococcal diseases (1-2), the immunogenicity of PPSV23 in COPD patients is not known for all 23 vaccine serotypes. COPD prevalence and immunosenescence both increase with age, making assessment of immunogenicity important to determine. An intervention cohort study was conducted to compare antibody geometric mean concentrations (GMCs) and 2-fold increases in antibody levels before and 4 weeks after administering a dose of PPSV23 to 89 COPD patients who were invited by respiratory physicians to the study and agreed to participate. We found that PPSV23 provided good immunogenicity, with 2-fold increases in antibody levels ranging from 65.2% to 94.4%, and significant increases in GMCs, but with little difference by age, presence of comorbidities, and COPD severity for most serotypes. Our findings support current recommendations to offer PPSV23 to COPD patients.
COPD represents an important public health challenge that is preventable and treatable. The disease burden of COPD is considered serious globally (1) and in China (3). Patients with COPD have frequent AECOPD that can lead to further decline in lung function, accelerating disease progression, increasing risk of death and family economic burden (4). Pneumococcus is 1 of the 3 most common bacterial antecedents to AECOPD (4). Vaccination with PPSV23 can prevent pneumococcal-related diseases.
This study was carried out in Tangshan City, Hebei Province, China between September and December 2019. Doctors from respiratory outpatient clinics of three hospitals (Majiagou, Linxi, and Kangfu) invited patients to participate in the study. Eligible patients had to have a ratio of post-bronchodilator one-second forced expiratory volume (FEV1) to forced vital capacity (FVC) of less than 0.70 (1), be less than 80 years of age, and have stable COPD. Patients who received PPSV23 in the past 5 years or had a history of allergy to any vaccine component were excluded.
Subjects received a single intramuscular injection of 0.5 mL of PPSV23 that was produced by Merck Sharp & Dohme Corporation. Venous blood samples were obtained from each subject before and 4 weeks after vaccination. Enzyme-linked immunosorbent assay (ELISA) was used to measure antibody responses for the 23 serotypes in accordance with the WHO recommendation protocol (5) by National Institute for Food and Drug Control in China. An antibody-fold-increase was calculated by dividing the post-vaccination antibody concentration by the pre-vaccination antibody concentration. GMCs and 2-fold increase rates were used as outcomes. One-half of the assay detection limit was assigned to results below the detection limit when calculating GMCs.
For the sample size calculation, the rate of 2-fold increases in antibody levels was assumed to be 70% (6) and that an acceptable 2-fold increase rate would be 50%; a one-sided significance test was used, α=0.05, and one-sided power=0.80 to determine the minimum sample size to be 73. The sample size was targeted to 88 to allow for a 20% loss of subjects to follow-up.
EpiData (version 3.1, EpiData Software, Epi Info V6, Denmark) and SAS (version 9.4, SAS Institute, Cary, NC, USA) were used for data entry and statistical analysis. Chi-squared or Fisher’s exact probability testing was used to compare 2-fold-increase rates. Student’s t-tests or analysis of variance was used to compare GMCs. P<0.05 was considered a statistically significant difference.
Overall, 95 subjects were enrolled and 89 subjects completed the study; 67 subjects (75.3%) were men; the median age was 64 years (range 47−77) and 46.1% were 65 years or older; 41 (46.1%) had co-morbid conditions (hypertension, diabetes, cardiovascular and cerebrovascular diseases, etc); 1 subject had received PPSV23 in the past. The distribution of COPD severity was 4 (4.5%) mild, 54 (60.7%) moderate, 30 (33.7%) severe, and 1 (1.1%) very severe.
The 2-fold increase rates ranged from 65.2% (serotype 3) to 94.4% (serotype 2). There were statistically significant differences in 2-fold increase rates among the 23 vaccine serotypes. Serotype 2 and 7F had the highest 2-fold increase rates (>90%); 2-fold increase rates for serotypes 1, 4, 5, 8, 9N, 9V, 10A, 12F, 15B, 17F, 18C, 19F, 20, 23F, and 33F were between 80% and 90%; 2-fold increase rates for serotype 11A, 19A, and 22F were between 70% and 80%; and 2-fold increase rates for serotypes 3, 6B, and 14 were less than 70%. The 2-fold increase rate for serotype 19F was higher among patients with mild or moderate-severity COPD (P=0.021) compared with patients with severe or very severe COPD. The 2-fold increase rates for 8, 10A, and 33F in patients with comorbidities were lower than for patients without comorbidities (P=0.005, P=0.016, P=0.01). There were no other statistically significant differences by age, COPD severity, or presence of comorbidities (Table 1, Figure 1).
Serotype Age group COPD severity Comorbidities Total <65 years ≥65 years Mild and
moderateSevere and
very severeYes No 1 89.6 (77.3−96.5) 82.9 (67.9−92.9) 87.9 (76.7−95.0) 83.9 (66.3−94.6) 80.5 (65.1−91.2) 91.7 (80.0−97.7) 86.5 (77.6−92.8) 2 95.8 (85.8−99.5) 92.7 (80.1−98.5) 96.6 (88.1−99.6) 90.3 (74.3−98.0) 92.7 (80.1−98.5) 95.8 (85.8−99.5) 94.4 (87.4−98.2) 3 68.8 (53.8−81.3) 61.0 (44.5−75.8) 62.1 (48.4−74.5) 71.0 (52.0−85.8) 63.4 (46.9−77.9) 66.7 (51.6−79.6) 65.2 (54.3−75.0) 4 85.4 (72.2−93.9) 82.9 (67.9−92.9) 84.5 (72.6−92.7) 83.9 (66.3−94.6) 78.1 (62.4−89.4) 89.6 (77.3−96.5) 84.3 (75.0−91.1) 5 85.4 (72.2−93.9) 85.4 (70.8−94.4) 86.2 (74.6−93.9) 83.9 (66.3−94.6) 80.5 (65.1−91.2) 89.6 (77.3−96.5) 85.4 (76.3−92.0) 6B 66.7 (51.6−79.6) 68.3 (51.9−81.9) 72.4 (59.1−83.3) 58.1 (39.1−75.5) 68.3 (51.9−81.9) 66.7 (51.6−79.6) 67.4 (56.7−77.0) 7F 93.8 (82.8−98.7) 87.8 (73.8−95.9) 91.4 (81.0−97.1) 90.3 (74.3−98.0) 87.8 (73.8−95.9) 93.8 (82.8−98.7) 91.0 (83.1−96.0) 8 89.6 (77.3−96.5) 87.8 (73.8−95.9) 87.9 (76.7−95.0) 90.3 (74.3−98.0) 78.1 (62.4−89.4) 97.9 (88.9−99.9) 88.8 (80.3−94.5) 9N 95.8 (85.8−99.5) 82.9 (67.9−92.9) 89.7 (78.8−96.1) 90.3 (74.3−98.0) 85.4 (70.8−94.4) 93.8 (82.8−98.7) 89.9 (81.7−95.3) 9V 89.6 (77.3−96.5) 85.4 (70.8−94.4) 89.7 (78.8−96.1) 83.9 (66.3−94.6) 85.4 (70.8−94.4) 89.6 (77.3−96.5) 87.6 (79.0−93.7) 10A 91.7 (80.0−97.7) 78.1 (62.4−89.4) 86.2 (74.6−93.9) 83.9 (66.3−94.6) 75.6 (59.7−87.6) 93.8 (82.8−98.7) 85.4 (76.3−92.0) 11A 77.1 (62.7−88.0) 70.7 (54.5−83.9) 74.1 (61.0−84.7) 74.2 (55.4−88.1) 75.6 (59.7−87.6) 72.9 (58.2−84.7) 74.2 (63.8−82.9) 12F 91.7 (80.0−97.7) 87.8 (73.8−95.9) 93.1 (83.3−98.1) 83.9 (66.3−94.6) 87.8 (73.8−95.9) 91.7 (80.0−97.7) 89.9 (81.7−95.3) 14 70.8 (55.9−83.1) 65.9 (49.4−79.9) 72.4 (59.1−83.3) 61.3 (42.2−78.2) 75.6 (59.7−87.6) 62.5 (47.4−76.1) 68.5 (57.8−78.0) 15B 85.4 (72.2−93.9) 90.2 (76.9−97.3) 87.9 (76.7−95.0) 87.1 (70.2−96.4) 82.9 (67.9−92.9) 91.7 (80.0−97.7) 87.6 (79.0−93.7) 17F 89.6 (77.3−96.5) 87.8 (73.8−95.9) 91.4 (81.0−97.1) 83.9 (66.3−94.6) 85.4 (70.8−94.4) 91.7 (80.0−97.7) 88.8 (80.3−94.5) 18C 93.8 (82.8−98.7) 80.5 (65.1−91.2) 89.7 (78.8−96.1) 83.9 (66.3−94.6) 82.9 (67.9−92.9) 91.7 (80.0−97.7) 87.6 (79.0−93.7) 19A 79.2 (65.0−89.5) 75.6 (59.7−87.6) 79.3 (66.7−88.8) 74.2 (55.4−88.1) 73.2 (57.1−85.8) 81.3 (67.4−91.1) 77.5 (67.5−85.7) 19F 83.3 (69.8−92.5) 78.1 (62.4−89.4) 87.9 (76.7−95.0) 67.7 (48.6−83.3) 75.6 (59.7−87.6) 85.4 (72.2−93.9) 80.9 (71.2−88.5) 20 83.3 (69.8−92.5) 80.5 (65.1−91.2) 82.8 (70.6−91.4) 80.7 (62.5−92.6) 80.5 (65.1−91.2) 83.3 (69.8−92.5) 82.0 (72.5−89.4) 22F 77.1 (62.7−88.0) 68.3 (51.9−81.9) 77.6 (64.7−87.5) 64.5 (45.4−80.8) 68.3 (51.9−81.9) 77.1 (62.7−88.0) 73.0 (62.6−81.9) 23F 85.4 (72.2−93.9) 80.5 (65.1−91.2) 82.8 (70.6−91.4) 83.9 (66.3−94.6) 82.9 (67.9−92.9) 83.3 (69.8−92.5) 83.2 (73.7−90.3) 33F 93.8 (82.8−98.7) 85.4 (70.8−94.4) 91.4 (81.0−97.1) 87.1 (70.2−96.4) 80.5 (65.1−91.2) 97.9 (88.9−99.9) 89.9 (81.7−95.3) Abbreviations: PPSV23=23-valent pneumococcal polysaccharide vaccine; COPD=chronic obstructive pulmonary disease. Table 1. Two-fold increase rates (%) of 23 serotypes and 95% confidence intervals after PPSV23 vaccination in COPD patients in Tangshan City, Hebei Province, China during September to December 2019.
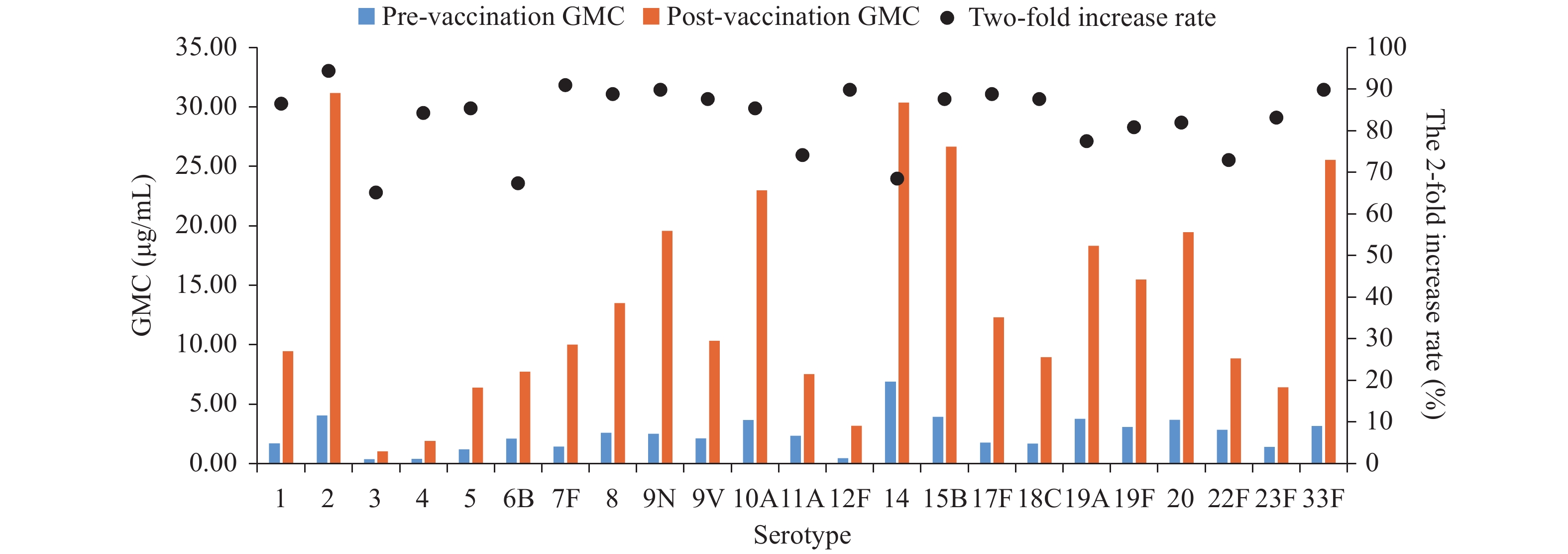 Figure 1.
Figure 1.Geometric mean concentrations (GMCs) pre- and post-vaccination and 2-fold increase rates of 23 serotypes post 23-valent pneumococcal polysaccharide vaccination in patients with chronic obstructive pulmonary disease in Tangshan City, Hebei Province, China during September to December 2019.
GMCs ranged from 0.38 μg/mL (serotype 3) to 6.90 μg/mL (serotype 14) at baseline and from 1.03 μg/mL (serotype 3) to 30.36 μg/mL (serotype 14) after vaccination. There were statistically significant differences in baseline GMCs (P<0.0001) and post-vaccination GMCs (P<0.0001) among the 23 serotypes. Compared with subjects ≥65 years, younger subjects had lower baseline GMCs for serotype 9V (P=0.035) and higher post-vaccination GMC for serotype 10A (P=0.030). After vaccination, GMCs of serotype 6B and 19F among patients with mild or moderate COPD were higher than among patients with severe and very severe COPD (P=0.04, P=0.039). There were no other significant differences in GMCs by age, COPD severity, or presence of comorbidities (Figure 1, Supplementary Table S1).
HTML
-
This study found that immunogenicity provided by 1 dose of PPSV23 in COPD patients was good. Although immunogenicity varied by serotype, it varied little by age, presence of comorbidities, and severity of COPD for most of the vaccine serotypes. This is the first study to assess immunogenicity of all 23 serotypes from one dose of PPSV23 in COPD patients. The findings support current recommendations to offer PPSV23 to COPD patients in China to provide protection from pneumococcal disease and prevent or reduce the number of AECOPD.
There have been some evaluations of PPSV23 immunogenicity in COPD patients. A study conducted in Taiwan, China assessed the immunogenicity of 8 PPSV23 serotypes (4, 6B, 7F, 9V, 14, 18C, 19F, and 23F) in COPD patients (6). Two-fold increases for serotypes 6B and 14 and post-vaccination GMCs of the 8 corresponding serotypes in this study were lower than that in the study conducted in Taiwan, China. Differences between the two studies may be due to different blood collection intervals (4 weeks vs. 6 weeks) or different ELISA methods.
Two studies conducted in Japan among COPD patients showed lower 2-fold increases and post-vaccination GMCs for 6B, 14, 19F, and 23F than in this study (7-8). Differences between the studies may have been due to different pre-vaccination antibody concentrations or different characteristics of subjects. In this study, pre-vaccination GMCs for serotype 6B, 19F, and 23F were lower than that in the study conducted in Japan (7). The median age in this study was younger and fewer comorbid illnesses were considered to be other respiratory diseases than that in the study conducted in Japan (8).
The immune response induced by PPSV23 in COPD patients appears to be comparable with those of age-matched healthy adults. One study assessed immunogenicity of PPSV23 in COPD patients and healthy controls and found that there were no statistically significant differences in the antibody GMCs between COPD patients and healthy controls (9). The 2-fold increase rates of 23 serotypes in this study were not lower than those in the Phase III licensure clinical trial of PPSV23 conducted in China (10).
This study was subject to at least four limitations. First, there were no healthy controls to assess differences in immune response between COPD patients and healthy adults. Other studies have shown comparable immunogenicity, however. Second, subjects were not selected randomly, which could affect representativeness. Third, the sample size was too small to assess immunogenicity among subgroups. Finally, the study was short term and did not provide evidence of persistence of immunity. There are several studies reporting 1−2 years of immune persistence following PPSV23 vaccination of COPD patients (6-7,9). Only one study reported long-term persistence (8). Following these subjects for an additional 4−5 years could provide additional evidence of persistence.
Antibody levels that correlate with protection from pneumococcal disease have not been clearly defined for adults. Thus, the relationship between immunogenicity and effectiveness is unclear. This important relationship merits additional research using experimental study designs to determine health and economic benefits of PPSV23 in COPD patients.
In conclusion, the immunogenicity of one dose of PPSV23 is good in COPD patients. PPSV23 vaccination should continue to be recommended for COPD patients in China.
Acknowledgments: Jianquan Li, Xiaohui Zhang, Yi Wang, Yanru Zhang, Yadi Su, Xueying Li, and other colleagues; Lance Rodewald, MD.
Conflicts of interest: We declare no competing interests. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
Funding: National Key R&D Program of China (2017YFC1309304).
| Citation: |



 Download:
Download:




