-
Brucellosis is one of the most common zoonotic diseases, caused by species of the genus Brucella, that affects domestic and farm livestock and a wide range of wild mammals (1–2). Endemic areas are primarily located in the low- and middle-income countries across the Mediterranean region, the Arabian Peninsula, Africa, Asia, and Central and South America, with major regional differences (3-5). The highest prevalence in animals was observed in countries of the Middle East and sub-Saharan Africa, China, India, Peru, and Mexico (3,6–7). Only a few countries in the world are free from the infectious agent and are mainly in developed regions in Western and Northern Europe, Canada, Japan, Australia, and New Zealand (1).
The human brucellosis, also known as undulant fever or Malta fever, was first recognized in Malta during the 1850s. The infection in humans is primarily caused by direct contact with infected cattle (Brucella abortus), sheep and goats (B. melitensis), pigs (B. suis), dogs (B. canis), or by ingesting unpasteurized and contaminated animal products. B. melitensis is the most common cause of reported human brucellosis cases and the most severe form of the disease (4). Human infections caused by inhaling airborne agents were also reported (8). In addition, because brucellosis is one of the most common laboratory-acquired infections, strict safety precautions should be followed when handling cultures and infected samples.
Human brucellosis affects humans of all ages and sex and typically manifests as a variety of symptoms and signs including intermittent or irregular fever, headache, weakness, profuse sweating, chills, weight loss, and general aching. These non-specific clinical manifestations make it difficult to distinguish from other febrile conditions. However, if the disease is not diagnosed properly and treated promptly, it may become chronic and persist for years, leading to complications such as osteoarticular, hepatobiliary, cardiovascular, and central nervous system diseases (9). In addition, the disease is regarded as an important occupational hazard to livestock workers. Veterinarians, farmers, and slaughterhouse workers are vulnerable as they handle infected animals and aborted fetuses or placentae.
Human brucellosis has been greatly controlled in many countries, and its epidemiological features have changed drastically over the past few decades due to the various improvements in sanitary conditions, socioeconomic development, political reasons, and the increasing domestic and international human mobility (4-6,10). However, considering the large number of cases reported across the world each year and its serious health consequences and socioeconomic impacts, human brucellosis remains a global public health challenge, especially in regions where animal infections are common.
Fox example, brucellosis is considered endemic in most Middle Eastern countries with a high number of human cases reported in Yemen, Iran, Syria, Turkey, and Saudi Arabia in recent years (6,11). The highest annual incidence rate was recorded in Yemen (88.6 cases/100,000 person-years), Syria (40.6/100,000), and Iran (18.6/100,000) in 2014–2017 (6). McDermott JJ et al. (2) found a high average prevalence (11%) among high-risk human populations in Africa and Asia, such as veterinarians, livestock handlers, and slaughterhouse workers, suggesting that brucellosis is an ongoing epidemic in African and Asian continents. Data on incidence of brucellosis are often underreported because the surveillance system relies on passive reports that collect information from hospitals and diagnostic laboratories, especially in areas with low capacities of healthcare and diagnosis. Therefore, the real burden of human brucellosis in low-income countries may be far greater than figures reported.
However, human brucellosis is also reported in some high-income countries where brucellosis has been eliminated or transmitted at a relative low level. For instance, 381 confirmed cases of brucellosis were reported by 28 European Union countries in 2017 with an overall rate of 0.09 cases/100,000 person-years (12). Greece, Italy, and Spain reported the highest numbers of confirmed cases, accounting for 67.2% of all cases. Greece had reported the highest incidence rate, followed by Italy and Portugal, as well as Spain and Sweden. In Sweden, all cases occurred in travelers from countries with ongoing epidemics.
In China, the epidemiological features of human brucellosis have significantly changed in the past 7 decades, especially during the period of dramatic socioeconomic changes since 1980 (10,13). Before 1950, brucellosis in both animals and humans was highly prevalent across the country. Since 1950, the activities for brucellosis prevention and control were gradually introduced in the mainland of China. During 1955–1979, human brucellosis was relatively steady with an incidence rate of 0.4–1.0 cases/100,000 person-years and peaked during 1957–1963 and 1969–1971 (13–14). After the implementation of a national control program (14), the incidence rate of human brucellosis decreased gradually since 1979 and reached its lowest level in 1994 (interquartile range: 0.05–0.10/100,000) (13).
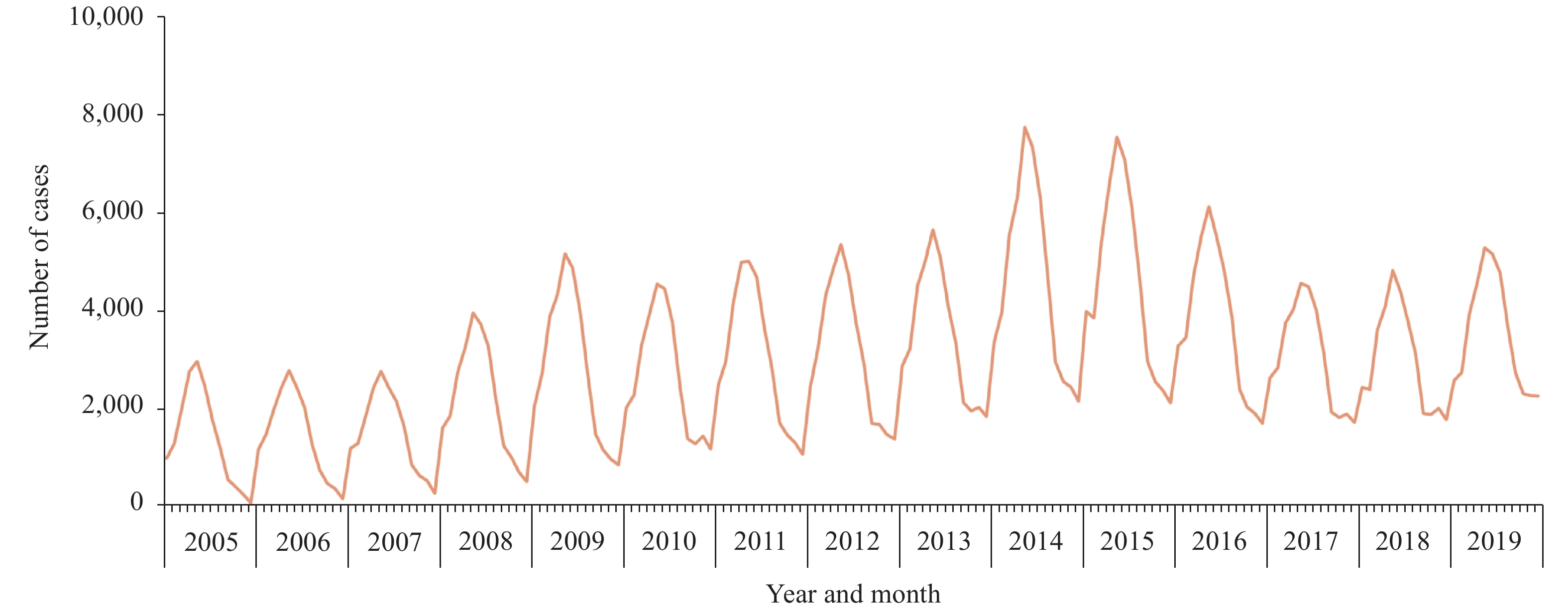
Nevertheless, human brucellosis has reemerged in mainland China since the mid-1990s (10,13), with the incidence increasing from 1995 and peaking at 57,222 cases in 2014 (4.2 cases/100,000 person-years) (Figure 1). Following the resurgence, this disease has also expanded geographically from northwestern to southeastern China (15). The affected regions gradually shifted from northwestern pasturing provincial-level administrative divisions (PLADs), including Inner Mongolia, Xinjiang, Xizang (Tibet), Qinghai, and Ningxia, through adjacent grasslands and agricultural areas with a high density of sheep and goats (16), to coastal PLADs and southeast China. In addition, seasonal patterns in human brucellosis were also found as most cases were reported between February and July (Figure 1), which were coincided with the peak period for abortions and parturitions among livestock in the spring and summer (17). This reemergence across China might be attributed to a variety of factors, such as the increasing demand for meat consumption, the expansion of animal industries, urbanization, the lack of hygienic measures and vaccination in animal husbandry, and the failure to remove infected animals.
The National Brucellosis Prevention and Control Plan (NBPCP) was implemented in 2016–2020, aiming to contain this disease in animals and humans across China (18). In this issue of the China CDC Weekly, four important studies about brucellosis have been published to understand the changing epidemiology and control progress of human brucellosis across China (19-22). Lin S et al. (19) investigated the serological prevalence among the high-risk population in brucellosis endemic areas in China in 2019–2020. They found that the seroprevalence decreased following the implementation of NBPCP in endemic areas compared to the reported rates in the previous years. However, an analysis conducted by Tao Z et al. (20) revealed that the number of human cases in China had decreased from 47,139 in 2016 to 37,947 in 2018, but then rebounded in 2019. The rebound was mainly related to the resurgence in Inner Mongolia, and most counties failed to meet the control targets of NBPCP, which is calling for improved strategies and more resources to ensure a sustainable brucellosis control program in China.
The reasons for recent rebound in China, especially in Inner Mongolia, might include: 1) the circulation and expanded transmission of Brucella among livestock led to an increase in human infections; 2) the improvement of active monitoring, diagnosis, and reporting of human brucellosis also contributed to the increasing number of detected cases; and 3) with recent increases in the price of beef and lamb, the number of people engaged in husbandry might have increased, whereas the awareness of brucellosis prevention still remained substandard, resulting in a surge of infected people. For example, the results of survey published by Wang Z et al. (21) revealed that the awareness of brucellosis knowledge and the utilization efficiency of personal protective equipment among the high-risk population remained relatively low compared to the target set in the NBPCP. More effective health education about brucellosis should be carried out to reduce the infection risk in high-risk population, and an efficient surveillance information system could provide timely data for assessing the effectiveness of control program and case management and follow-up. As part of the effort, Wulanchabu City in Inner Mongolia had been establishing a Brucellosis Integrated Information System (BIIS) since the year of 2013, the first human brucellosis-specific reporting system in China, and Dong S et al. (22) have evaluated the performance of BIIS on case report and management, providing important evidence for continuously improving the system.
Brucellosis remains a significant health threat in many countries including China (7). As there is no human vaccine against brucellosis, the most effective prevention strategy is the elimination of infections in animal hosts in combination with raising protections, food-safety measures, occupational hygiene, and laboratory safety (23). Vaccinating cattle, goats, and sheep are also recommended in areas with high prevalence. Most importantly, the One Health approach (24), involving collaborative efforts for health in humans, animals, and the environment as well as multiple other sectors, is vital to monitor disease transmission and to mitigate health and socioeconomic impacts of brucellosis, eventually eliminating the disease in human and animal populations across the world.
Funding: National Science and Technology Major Project of China (2018ZX10713001-001); The National Natural Science Fund of China (81773498); National Science and Technology Major Project of China (2016ZX10004222-009).
HTML
| Citation: |



 Download:
Download:




