-
Introduction: Late HIV diagnosis represents a critical public health challenge among older adults in China. Identifying its correlates is essential for enhancing timely detection and improving health outcomes.
Methods: We analyzed newly reported human immunodeficiency virus (HIV)-infected cases aged ≥50 years using national surveillance data from 2022–2024. Logistic regression was employed to examine factors associated with late diagnosis, while decision tree modeling captured complex variable interactions.
Results: Among 162,026 cases, 77.78% were diagnosed late. Late diagnosis was more frequently observed among males, older individuals, non-migrants, ethnic minorities, those without sexually transmitted disease (STD) history, individuals with higher education, cases diagnosed in medical institutions, residents of eastern China, and those infected through non-marital commercial heterosexual contact (adjusted odds ratio=1.05–1.60). The decision-tree model identified transmission route and region as primary stratifiers. Within the eastern China branch, two terminal subgroups exhibited particularly high proportions of late diagnosis: individuals infected through non-marital commercial heterosexual contact (74.6%) and those infected through other heterosexual or men who have sex with men (MSM) routes who were diagnosed in medical institutions (73.0%).
Conclusions: Late HIV diagnosis among older adults in China remains persistently high. These findings identify specific population subgroups and diagnostic settings with elevated proportions of late diagnosis and underscore the need for targeted screening interventions, strengthened testing protocols in medical settings, and enhanced risk awareness among older heterosexual adults.
-
Late human immunodeficiency virus (HIV) diagnosis remains a major global challenge, despite substantial progress in HIV prevention and treatment (1). The European Late Diagnosis Consensus Working Group (2) defines late HIV diagnosis as an initial HIV diagnosis with a CD4 count <350 cells/μL or the presence of an acquired immunodeficiency syndrome (AIDS)-defining event, regardless of CD4 level. This phenomenon is particularly prevalent among adults aged ≥50 years, who often underestimate their infection risk and may engage in high-risk sexual behaviors without seeking timely testing (3). Among older adults, late diagnosis leads to compromised immune recovery, increased susceptibility to opportunistic infections, and elevated mortality. At the population level, delayed diagnosis undermines the public health benefits of Treatment as Prevention, increases healthcare expenditures, and heightens the risk of ongoing transmission (4). Although numerous studies have examined late HIV diagnosis, most have been regional or population-specific, leaving a gap in recent national-level evidence. Furthermore, prior national investigations have predominantly focused on individual risk factors without exploring interaction-based risk stratification among older adults. To address these limitations, we analyzed recent nationwide surveillance data from adults aged ≥50 years using a classification and regression tree (CRT) model. Our objectives were to identify high-risk subgroups through interaction-based analysis and to characterize factors associated with late diagnosis, thereby informing more targeted screening and intervention strategies.
Data were obtained from the Chinese HIV/AIDS Comprehensive Response Information Management System. Among newly reported cases from January 1, 2022 to December 31, 2024 with a final review status of “approved” and aged ≥50 years at diagnosis, late diagnosis was defined using five established criteria (5): 1) HIV/AIDS deaths from non-accidental causes; 2) surviving or accidental-death HIV/AIDS cases with CD4 count <350 cells/μL; 3) surviving or accidental-death AIDS cases with CD4 count between 350–499 cells/μL; 4) surviving or accidental-death AIDS cases without CD4 testing; 5) surviving or accidental-death HIV cases without CD4 testing. For the fifth category, late diagnosis status was estimated based on the proportion of CD4 <350 cells/μL among tested cases within the same demographic stratum. Because this estimation approach could not be verified at the individual level and introduced potential measurement error, we excluded the fifth category from the multivariable analysis to ensure diagnostic accuracy.
Regions were grouped into eastern, central, and western zones according to economic development level. The variable ‘source of detection’ was included as a surveillance-based classification to capture differences in case-finding contexts. “Transmission route” was classified using standardized categories from the national HIV surveillance system at the time of case reporting. Variables were selected for analysis based on two criteria: their established relevance in prior surveillance-based studies of late HIV diagnosis and their availability within the national HIV surveillance system with standardized reporting protocols. Data were cleaned using Excel 2021 (Microsoft Corporation, Redmond, Washington, USA) and analyzed in R version 4.2 (version 4.2, R Foundation for Statistical Computing, Vienna, Austria). Chi-square tests were used for group comparisons, and logistic regression was applied for both univariate and multivariate analyses. Adjusted odds ratios (aORs) and 95% confidence intervals (CIs) were calculated. Statistical significance was set at P<0.05. A classification and regression tree (CRT) model was developed to predict late diagnosis using recursive binary splitting, which partitions data based on variables that maximize homogeneity within resulting subgroups (measured by reduction in Gini impurity, a metric quantifying classification accuracy). Data were randomly divided into training (70%) and validation (30%) sets, with cross-validation pruning applied to prevent overfitting. Variable importance was assessed by evaluating each variable’s split frequency and contribution to model performance, with region and transmission route emerging as key stratifiers.
A total of 162,026 newly reported HIV/AIDS cases aged ≥50 years were included in the analysis, of whom 71.13% were male (115,279/162,026). Overall, 77.78% (126,018/162,026) were diagnosed late. Demographic characteristics revealed that nearly half were aged 50–59 years (47.09%), 65.0% were farmers, and the majority were of Han ethnicity (87.93%), had education below primary school level (61.45%), and were married (57.10%). Geographically, eastern China accounted for 48.45% of all cases, and 96.19% were non-migrants. Heterosexual transmission was the predominant route (90.23%), with non-marital non-commercial contact representing the most common subtype (42.03%, 68,106/162,026). Most cases were identified through medical institutions (69.51%, 112,619/162,026).
After excluding individuals without CD4 results, 145,741 cases remained for multivariable analysis. Among these cases with complete CD4 data, 75.29% (109,733/145,741) were diagnosed late, a slightly lower proportion than the overall sample due to the exclusion of estimated late diagnoses without laboratory confirmation. Late diagnosis demonstrated positive associations with male sex (aOR=1.13), non-migration status (aOR=1.18), absence of STD history (aOR=1.11), higher educational attainment (aOR=1.08–1.10), and residence in eastern China (aOR=1.14). Weaker positive associations were observed for ethnic minority status (aOR=1.06) and older age (aOR=1.08–1.11). Regarding transmission routes, non-marital commercial heterosexual contact exhibited the highest risk relative to injection drug use (IDU) (aOR=1.60, 95% CI: 1.25, 2.05). Cases detected in medical institutions also demonstrated elevated odds of late diagnosis compared with those identified through key population screening (aOR=1.05, 95% CI: 1.02, 1.08) (Table 1).
Variables Late diagnosis Multivariate analysis N Proportion (%) β S.E. Z P aOR (95% CI) Sex Female 30,393 70.51 1.00 (Reference) Male 79,340 77.30 0.13 0.01 8.90 <0.05 1.13 (1.10, 1.16) Age (years) 50–59 50,996 76.08 1.00 (Reference) 60–69 35,961 80.54 0.10 0.01 8.28 <0.05 1.11 (1.08, 1.13) 70–79 19,092 78.34 0.08 0.02 5.15 <0.05 1.08 (1.05, 1.11) ≥80 3,684 73.34 0.03 0.03 −4.87 <0.05 0.87 (0.82, 0.92) Migration status Yes 3,932 75.27 1.00 (Reference) No 105,801 76.02 0.17 0.03 6.05 <0.05 1.18 (1.12, 1.25) Occupation Farmer 71,656 75.64 1.00 (Reference) Others 38,077 74.64 −0.03 0.01 −2.22 <0.05 0.97 (0.95, 0.99) Ethnicity Han 96,299 74.31 1.00 (Reference) Ethnic minorities 13,434 75.43 0.05 0.02 3.16 <0.05 1.06 (1.02, 1.09) History of sexually transmitted diseases (STDs) Yes 14,126 75.08 1.00 (Reference) No 87,098 75.35 0.10 0.02 6.63 <0.05 1.11 (1.08, 1.14) Unknown 8,509 75.05 0.07 0.02 3.06 <0.05 1.08 (1.03, 1.13) Education level Illiterate 12,952 74.08 1.00 (Reference) Primary school 54,191 75.06 0.09 0.02 5.13 <0.05 1.09 (1.06, 1.13) Junior high school 31,131 75.85 0.10 0.02 4.98 <0.05 1.10 (1.06, 1.14) Senior high school or above 11,459 74.63 0.07 0.02 3.04 <0.05 1.08 (1.03, 1.13) Source of detection Key population screening 21,291 71.75 1.00 (Reference) Medical institutions 76,576 76.99 0.05 0.01 3.43 <0.05 1.05 (1.02, 1.08) Others 11,866 71.49 −0.03 0.02 −1.26 0.207 0.97 (0.94, 1.01) Regional distribution Western 51,944 74.12 1.00 (Reference) Central 17,544 74.78 0.04 0.02 2.49 <0.05 1.06 (1.03, 1.09) Eastern 40,245 76.49 0.13 0.01 10.38 <0.05 1.14 (1.11, 1.17) Route of transmission Injecting drug use 157 72.59 1.00 (Reference) Others or unknown 998 70.66 −0.26 0.13 −1.92 0.055 0.77 (0.59, 1.01) Male-to-male sexual contact 9,407 73.42 0.33 0.13 2.63 <0.05 1.40 (1.09, 1.79) Unclassified heterosexual transmission 5,451 71.04 −0.18 0.13 −1.40 0.161 0.84 (0.65, 1.07) HIV-positive spouse or regular partner 8,747 75.21 0.37 0.13 2.91 <0.05 1.45 (1.13, 1.86) Non-marital commercial heterosexual contact 39,201 77.92 0.47 0.13 3.73 <0.05 1.60 (1.25, 2.05) Non-marital non-commercial heterosexual contact 45,772 74.81 0.34 0.13 2.72 <0.05 1.41 (1.10, 1.80) Abbreviation: aOR=adjusted odds ratio; CI=confidence interval; S.E.=standard error; Z=Z statistic. Table 1. Factors associated with late diagnosis among cases aged ≥50 years, 2022–2024 (n=145,741).
The decision-tree model identified transmission route, region, migration status, sex, and source of detection as the primary stratifiers of late diagnosis, yielding 14 terminal subgroups (referred to as “nodes” in tree-based classification). The overall proportion of late diagnosis at the root node was 67.7%. Following stratification by region, the model identified two particularly high-risk terminal nodes within the eastern China branch (Node 3). The first high-risk pathway comprised individuals in eastern China infected through non-marital commercial heterosexual contact (Node 7), with a late diagnosis proportion of 74.6%. The second high-risk pathway included individuals in eastern China infected through transmission from an HIV-positive spouse/regular partner, men who have sex with men (MSM), or non-marital non-commercial heterosexual contact, who were subsequently diagnosed in medical institutions (Node 11), with a late diagnosis proportion of 73.0% (Figure 1).
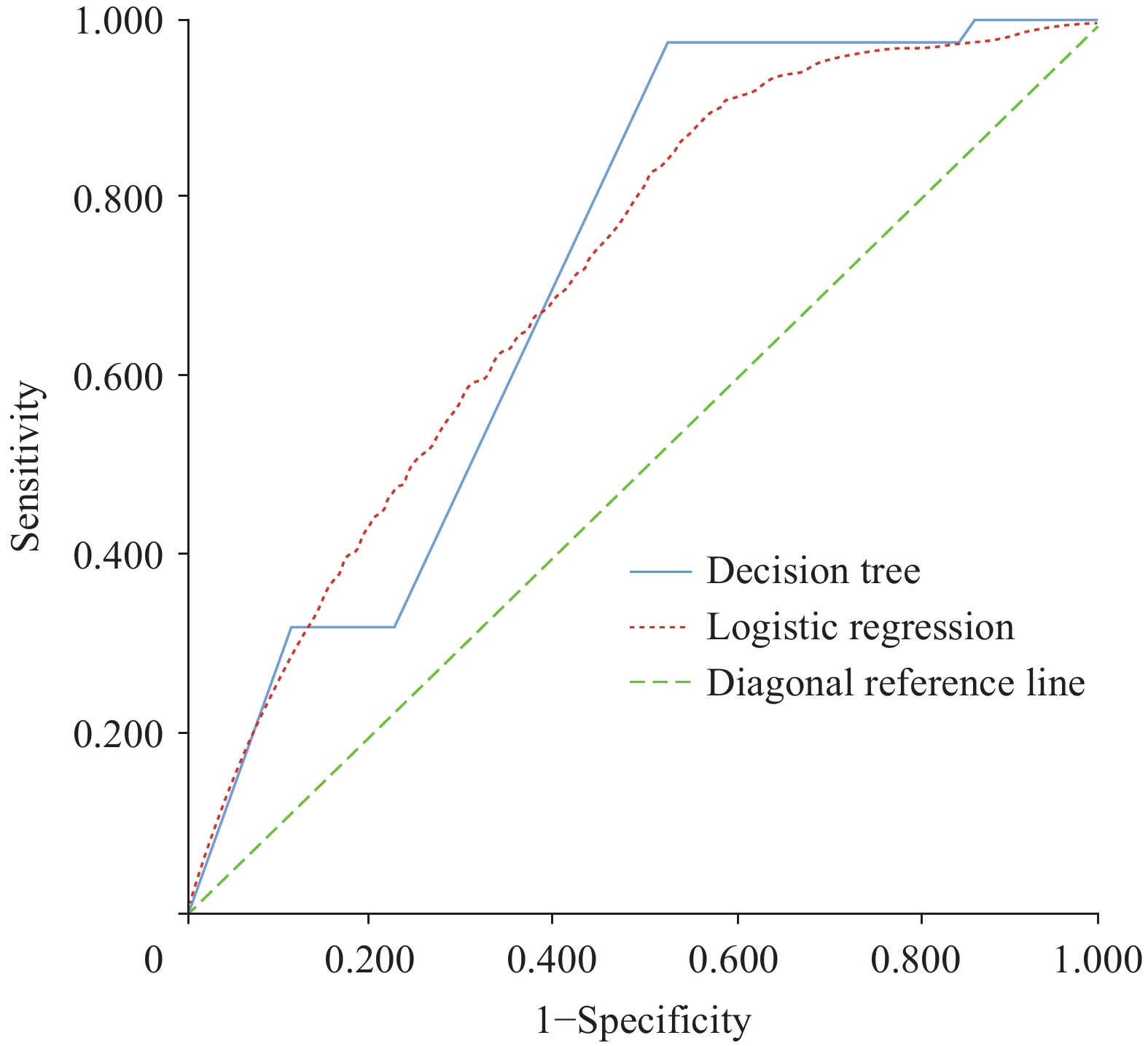
The logistic regression and decision-tree models demonstrated comparable overall discrimination, with AUCs of 0.710 (95% CI: 0.708–0.713) and 0.718 (95% CI: 0.715–0.721), respectively (P<0.001). However, the decision-tree model exhibited superior sensitivity (0.976 vs. 0.859) and a larger Youden index (0.451 vs. 0.326), indicating moderately better performance in identifying late diagnosis among adults aged ≥50 years (Figure 2).
-
Based on nationwide surveillance data from 2022 to 2024, a substantial proportion of adults aged ≥50 years were diagnosed at a late stage (77.78%), a rate that has remained persistently elevated compared with earlier reports in which late diagnosis consistently exceeded 65% during 2015–2019 (3). Although internal data suggested a temporary decline during 2020–2021, potentially attributable to coronavirus disease 2019 (COVID-19) pandemic-related disruptions in testing services, the persistently high proportion observed in recent years indicates that timely HIV diagnosis among older adults continues to present significant challenges. Previous studies have generally demonstrated that late HIV diagnosis among older adults is associated with a range of demographic and epidemiological factors, including male sex, heterosexual transmission, and facility-based diagnosis, most often identified through logistic regression analyses (3,6). Consistent with this literature, our regression results confirmed similar associations. Through decision-tree modeling, we extended these findings by illustrating how such factors co-occur within the observed data. Beyond identifying independent associations, the decision-tree model delineated two high-risk terminal nodes in eastern China — non-marital commercial heterosexual transmission and other heterosexual or MSM transmission routes combined with diagnosis in medical institutions — thereby uncovering interaction-based risk patterns not captured by regression analysis alone.
Notably, the eastern region exhibited the highest proportion of late diagnosis, contrasting with earlier studies that reported greater risks in southwestern China (7-8). This regional difference likely reflects variation in HIV service delivery pathways. Long-standing high-burden areas in the southwest have developed more established surveillance systems and proactive testing practices, whereas routine HIV screening remains less systematically integrated into outpatient care across many eastern settings (9). Additionally, a higher proportion of late diagnosis was observed among cases detected in medical institutions, suggesting persistent reliance on symptom-driven testing rather than routine screening protocols. Non-migrants demonstrated a higher proportion of late diagnosis, potentially reflecting reduced access to testing opportunities or support services. The variable “no history of sexually transmitted disease” showed only weak associations and did not emerge as a splitting variable in the decision-tree model, indicating limited contribution to risk stratification. While earlier studies predominantly identified individual-level factors such as sex, education, and transmission route (3,8), the decision-tree analysis in this study further delineated specific factor combinations — particularly heterosexual transmission in eastern regions combined with diagnosis in medical institutions — thereby providing a more nuanced characterization of heterogeneity in late diagnosis patterns.
This study has several limitations. First, reliance on case-reporting data without serological markers for recent infection (e.g., limiting antigen avidity assays) may introduce misclassification bias in distinguishing recent from long-standing infections at the time of diagnosis. Although excluding estimated cases reduced measurement error, this approach may have also reduced statistical power and limited generalizability. Second, potential misclassification of sexual transmission categories may exist (10), as self-reported behavioral information is susceptible to recall bias and non-disclosure. Third, the cross-sectional design precludes causal inference. Furthermore, key behavioral, psychosocial, and healthcare access–related factors were not captured in the surveillance dataset and therefore could not be assessed. Despite these limitations, the use of nationwide surveillance data and complementary analytic approaches strengthens the robustness of the findings. In conclusion, this study identified substantial heterogeneity in late HIV diagnosis among older adults in China, providing epidemiologically grounded evidence to inform targeted early testing strategies and prioritize interventions for high-risk subgroups.
-
Approval by the Ethics Committee of the National Center for AIDS/STD Control and Prevention, Chinese Center for Disease Control and Prevention (approval number: KX250108838).
HTML
| Citation: |




 Download:
Download: