HTML
-
Introduction: Measles is the third most common infectious disease, after Smallpox and Polio, and the global health community has committed to eliminating it. Recently, measles recurrence and outbreaks have occurred in several countries, posing a significant challenge for China, which is on the brink of eliminating measles. This study aimed to analyze the genetic characteristics of the D8 genotype of the measles virus (MeV) in Gansu Province in 2024 and provide a scientific basis for measles control and elimination efforts.
Methods: Nucleic acid-positive throat swab specimens were collected from measles cases confirmed in 14 municipal measles/rubella network laboratories in Gansu Province in 2024. MeV RNA was directly extracted using a viral nucleic acid extraction kit, and 634 nucleotides at the 3'-terminal of the nucleoprotein gene were amplified using one-step reverse transcription-polymerase chain reaction (RT-PCR). The amplified products were subjected to nucleotide sequencing to characterize the MeV gene.
Results: A total of 120 sequences of a 450-nucleotide region within the nucleoprotein gene (N-450) of MeV were obtained from the Measles and Rubella Network Laboratory of Gansu Province in 2024, of which 117 sequences were of the D8 genotype and 3 sequences were of the A genotype. The similarities in nucleotide and amino acid sequences between the D8 genotype sequences were 96.4%–99.1% and 96.7%–98.0%, respectively. The Gansu D8 sequences belonged to the same major branch as the D8 reference strain identified by the World Health Organization (WHO), which further divided into Cluster 1 and Cluster 2. By aligning oligonucleotide sequences using the real-time RT-PCR kit distributed by the Global Measles and Rubella Laboratory Network (GMRLN) with sequences of the D8 genotype from Gansu Province, this study discovered that every sequence in Cluster 1 occurred at the reverse primer annealing site, each containing three T-to-C transitions.
Conclusions: The cases detected in Gansu in 2024 were likely imported or linked to importation. It is recommended to continue vaccination programs with measles-containing vaccines in key areas and to carry out highly sensitive etiological monitoring and detection to provide data support for subsequent measles elimination efforts.
Measles is a highly contagious acute infectious respiratory disease caused by the measles virus (MeV) and characterized by fever and rash. Since the 1960s, widespread global immunization has significantly reduced the incidence of measles, with 83 countries achieving or maintaining the elimination of measles by the end of 2022. However, no World Health Organization (WHO) region has sustained elimination and countries such as the Czech Republic, Albania, and Mongolia have experienced a resurgence after being declared measles-free (1–2). China, having introduced the measles vaccine in 1978, reported a historically low incidence in 2022, yet cases have risen again since 2023, compounded by outbreaks in neighboring countries such as Vietnam, Kyrgyzstan, and Kazakhstan (3–5).
MeV has only one serotype but is classified into 8 groups and 24 genotypes based on genetic differences in hemagglutinin and nucleoprotein (6). The genotype distribution varies by region and period (7), and since 2018, only B3, D4, D8, and H1 have circulated globally (8–10). In Chinese mainland, H1a has been the dominant indigenous genotype over the past three decades, the along with vaccine-associated genotype A and imported genotypes, including B3, D4, D8, D9, D11, G3, and H2 (11).
In Gansu Province, surveillance from 2005 to 2019 only detected H1a, whereas only genotype A was detected from 2020 to 2023 (12). The D8 genotype was detected for the first time in 2024. This study aimed to analyze the molecular characteristics of the D8 genotype found in Gansu Province in 2024, provide a scientific basis for the control and elimination of measles, facilitate the subsequent development of measles prevention and control strategies, and help achieve the goal of eliminating measles.
Following the requirements of the National Measles Surveillance Program (13), 14 municipal measles and rubella network laboratories in Gansu Province performed real-time quantitative polymerase chain reaction (qPCR) testing for MeV nucleic acids in throat swabs or urine samples collected from suspected measles cases in 2024. All specimens positive for MeV nucleic acids were forwarded to the provincial measles and rubella reference laboratory within a designated timeframe for genotype identification, viral isolation, and culture. The case data in this study were obtained from the Chinese Information System for Disease Control and Prevention and included according to the present address and date of disease onset.
In 2024, 186 MeV nucleic acid-positive samples were reported in Gansu Province, all of which were throat swabs. All MeV-positive throat swab specimens were subjected to virus dissociation culture using Vero cells transfected with human SLAM molecules (Vero/hSLAM). Simultaneously, RNA extraction was performed on all samples using a nucleic acid extraction or purification kit (Cat No: SDK60104; Shuo Shi Biotechnology Co. Ltd.) following the kit instructions. The 634 nucleotides at the 3’-terminal of the nucleoprotein gene were amplified using the TaKaRa Prime Script One Step RT-PCR Kit Ver.2 (Dye Plus) (Cat No: RR057A) and specific primers (7). The amplification products were identified using capillary electrophoresis, and positive products were sent to Shanghai Berger Medical Co. for Sanger sequencing.
The raw sequences were trimmed using Lasergene DNAstar 7.1, and the reference sequences of the MeV nucleoprotein genes recognized by the WHO were obtained from GenBank. Homology analyses of nucleotide and amino acid sequences were performed using MEGA 11.0 (Pennsylvania State University, University Park, USA) and BioEdit 7.1 (Tom Hall, Carlsbad, USA) to construct a phylogenetic tree, using the neighbor-joining method, with statistical support assessed by bootstrap analysis based on 1,000 replicates (7,14). All sequences used in this study were submitted to the GenBank database under accession numbers YBB99888-YBB99999, YBC00001-YBC00008.
In 2024, the reported incidence of measles in Gansu Province was 0.90/100,000, a 10.25-fold increase from that in 2023. Compilation of the case data indicated that 902 measles surveillance cases were reported in Gansu Province in 2024, of which 221 were laboratory-confirmed measles cases (according to the Measles Prevention and Control Program (15), 3 vaccine-associated A genotype cases were excluded) distributed across 11 cities or states in Gansu Province. The highest number of confirmed cases were found in Linxia (33.94%), Lanzhou (23.08%), Gannan (17.65%), and Wuwei (9.95 %); less than 5.00% of the confirmed cases was found in other regions. Among the laboratory-confirmed measles cases, 186 were positive for MeV nucleic acids, 199 were positive for MeV-specific IgM antibodies, and 166 were double-positive for nucleic acids and serum IgM antibodies. Of the 186 MeV nucleic acid-positive samples, 34 measles virus strains were successfully isolated using Vero/hSLAM cell culture, all of which were transported to the National Measles Laboratory at the Chinese Center for Disease Control and Prevention. Sanger sequencing was performed on all 186 MeV nucleic acid-positive samples, resulting in 120 MeV N-450 sequences. Phylogenetic tree analysis showed that 117 sequences belonged to genotype D8 and three belonged to genotype A. The original sequences were rechecked in the measles room of the Virus Disease Institute of the Chinese Center for Disease Control and Prevention, and the standard naming of the wild-type strains was reported.
Based on the 120 sequences of a 450 nucleotide region within the nucleoprotein gene (N-450) analyzed in this study, 24 reference strains of MeV genotypes recommended by the WHO and named strains of the D8 genotype were selected to construct a phylogenetic tree (16). The results showed that the 117 D8 genotype sequences found in Gansu Province belonged to the same large branch as the D8 reference strain MVi/Manchester.GBR/30.94D8 (Figure 1) and were divided into two subgroups: Cluster 1 and Cluster 2. Cluster 1 was highly homologous to strain MVs/Almaty. KAZ/10.23, with a nucleotide similarity of 91.8% to 100.0%, whereas Cluster 2 more closely related to the named strains MVs/Thiruvananthapuram. IND/18.12 and MVs/Samut_Sakhon.THA/49.16, with nucleotide similarities ranging from 98.9% to 99.6%. The results also revealed that the Gansu D8 strain exhibited a relatively distant genetic relationship with the D8 strains circulating in China between 2012 and 2020 (such as MVi/Villupuram. IND/03.07, Southern Finland. FIN/49.18, and MVs/GirSomnath. IND/42.16) in the phylogenetic tree, suggesting that Gansu D8 belongs to a transmission chain unique to China. The 117 N-450 sequences of the D8 genotype differed from each other by 0–7 bases, with nucleotide sequence similarity ranging from 97.3% to 100.0% and amino acid sequence similarity ranging from 98.7% to 100.0%.
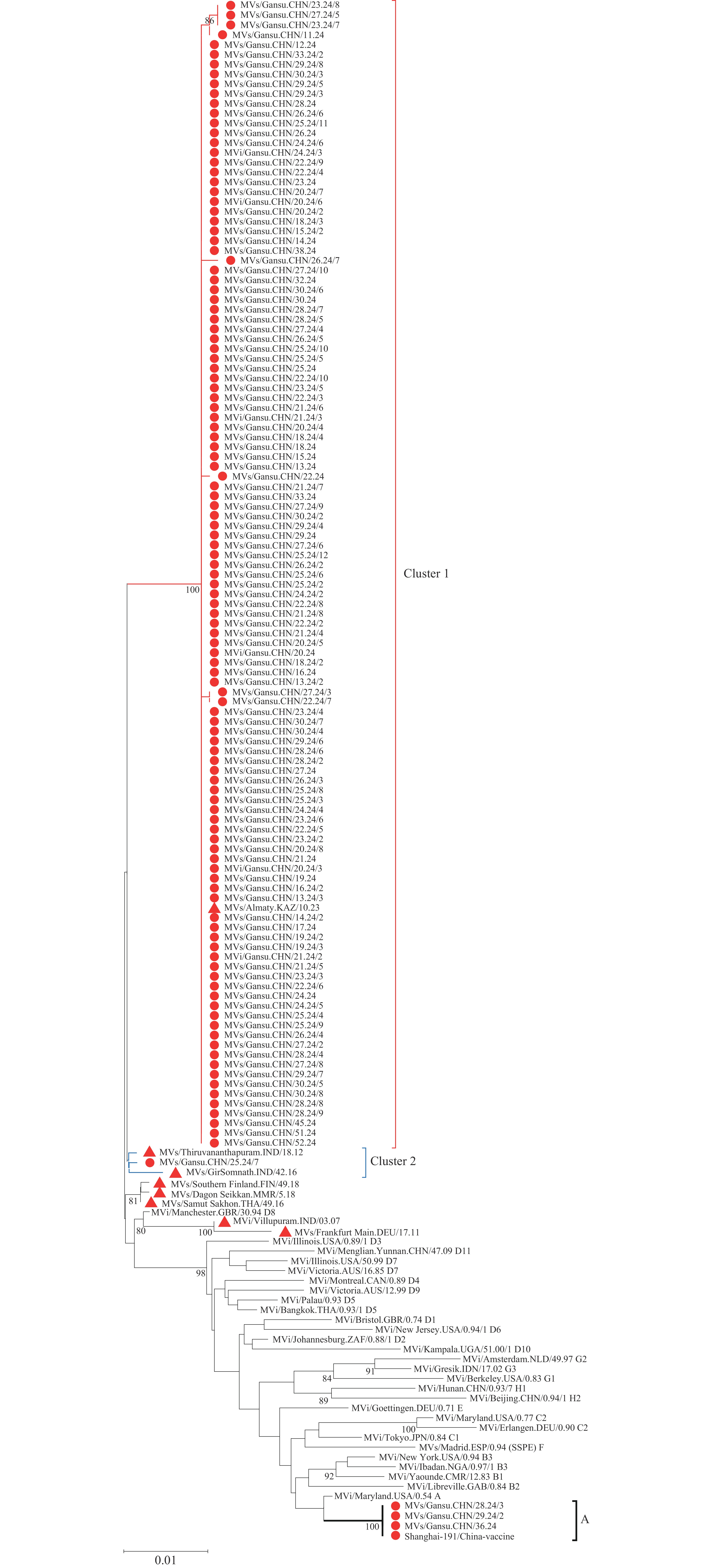 Figure 1.
Figure 1.Phylogenetic tree of the sequences of measles virus in Gansu Province in 2024 and World Health Organization reference strains (24 genotypes) and D8 genotyped named strains (based on sequence analysis of the N-450).
Note: sequences in Gansu Province in 2024 and the named strains of genotype D8 are indicated by red dots and red triangles, respectively, and significant bootstrapped values (>80) are indicated.Further analysis of the mutation sites within the 150 amino acid sequences in the N-450 region of the Gansu D8 sequences revealed that compared to the WHO D8 reference strain, Cluster 1 exhibited 4-5 common amino acid mutation sites (Table 1). L467P, T469A, G509S, and N522D were identified as the common mutations. Additionally, the three sequences were represented as MVs/Gansu.CHN/23.24/7 had the D461G mutation, and two sequences were represented by MVs/Gansu.CHN/27.24/3 strains had the G431R mutation; MVs/Gansu.CHN/22.24 and MVs/Gansu.CHN/26.24/7 had I504T and A478V mutations, respectively. In contrast to the WHO D8 reference strain, Cluster exhibited three mutation sites, G509S and N522D, consistent with Cluster 1, along with L467I. When comparing the Gansu D8 sequences with the vaccine reference strain Shanghai-191/China-vaccine, clusters 1 and 2 exhibited 18-19 amino acid mutations. Of these, 18 mutations, including K405R, I406T, K441R, and Y451N, were common and the remaining mutations were identical to those in the WHO D8 reference strain. Overall, these mutations occur outside α-MoRE (amino acids 488–499), therefore they are not expected to affect the phosphoprotein binding mediated by this domain.
Branch Representative Sequence Number of sequences Amino acid mutation sites 405 406 431 441 451 456 459 461 467 469 470 473 475 478 479 482 504 505 509 516 518 522 MVi/Manchester.GBR/
30.94D8R T G R N S L D L T S L I A S G I L G R Y N Shanghai-191/
China-vaccineK I R K Y P A D L T G P T A P S I S G T H N Cluster 1 MVs/Gansu.CHN/12.24 109 R T G R N S L D P A S L I A S G I L S R Y D MVs/Gansu.CHN/22.24 1 R T G R N S L D P A S L I A S G T L S R Y D MVs/Gansu.CHN/26.24/7 1 R T G R N S L D P A S L I V S G I L S R Y D MVs/Gansu.CHN/23.24/7 3 R T G R N S L G P A S L I A S G I L S R Y D MVs/Gansu.CHN/27.24/3 2 R T R R N S L D P A S L I A S G I L S R Y D Cluster 2 MVs/Gansu.CHN/25.24/7 1 R T G R N S L D I T S L I A S G I L S R Y D Abbreviation: MVs=measles virus sequence; WHO=World Health Organization. Table 1. Alignment of 150 amino acid sequence variant sites (amino acid 376–525) in the N-450 region among measles virus genotype D8 strains from Gansu Province, China; the vaccine strain; and the WHO D8 reference strain.
Furthermore, by aligning the oligonucleotide sequences of the real-time RT-PCR (rRT-PCR) kit distributed by the Global Measles and Rubella Laboratory Network (GMRLN) with the N-450 region of D8 strains from Gansu Province (17), this study found that the nucleotide variant sites in Cluster 1 occurred at the reverse primer annealing site, featuring three T-to-C transitions (Figure 2).
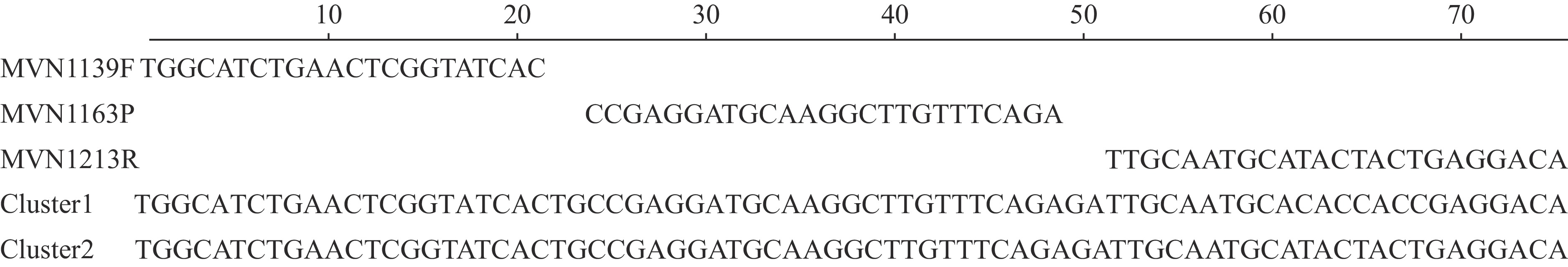 Figure 2.
Figure 2.Alignment of the GMRLN measles rRT-PCR primers and probe with the D8 genotype measles strains from Gansu Province within a 75-nucleotide region of the measles N gene.
Note: MVN1139F: forward primer; MVN1163P: probe; MVN1213R: reverse primer (shown as reverse complement). Abbreviation: GMRLN=Global Measles and Rubella Laboratory Network; rRT-PCR=real-time reverse transcription-polymerase chain reaction.
-
In 1998, the WHO established a genotype nomenclature system for MeV, and D8 was recognized as a branch of the D genotype. The first MeV D8 genotype sequence was reported in the United Kingdom in the 20th century and was subsequently reported globally (18). In the early 2000s, the D8 genotype was predominantly detected in Southeast Asia and Africa. By the 2010s, it was detected in measles outbreaks in several countries, with an extended distribution in Africa, Asia, Europe, and America. Data from the GMRLN indicate a decrease in the number of reported measles genotypes globally from nine in 2013 to two (B3 and D8) in 2021, with a notable upward trend in the proportion of reports of the D8 genotype: up from 34% in 2021 to 53% in 2022 and further to 74% in 2023 (8,18). These data suggest that the D8 genotype exhibits a continuing trend of global proliferation and gradual dominance.
Since 2009, the D8 genotype has gradually become one of the major imported genotypes in China and has been imported consistently from 2012 to 2020. It has been associated with three modes of transmission in China: sporadic importation, non-endemic transmission (<12 months), and endemic transmission (>12 months) (7). The D8 MeV genotype was not detected in Gansu Province before 2024. The D8 sequences of MeV detected in Gansu in 2024 were similar to those of the D8 strains detected in China between 2012 and 2020 (including MVi/Villupuram. IND/03.07, Southern Finland. FIN/49.18, and MVs/GirSomnath. IND/42.16), but they were distantly related in the phylogenetic tree (Figure 1), suggesting that the Gansu Province D8 sequences may occur in different transmission chains. The representative N-450 sequences of Gansu were compared using BLAST in the National Center for Biotechnology Information database, and the representative sequence was found to be MVs/Gansu. CHN/27.24 in Cluster 1, similar to that of MVs/Milano. ITA/3.24 and MVs/Sarajevo. BIH/04.24, with a sequence similarity of 100.00%. Its similarity with the other representative sequences was 99.56% –99.78%. Sequence similarity between Cluster 2 and the American MVs/Hawaii. USA/13.23 strain was 100.00%. These molecular epidemiological data suggest that the D8 strain detected in Gansu Province may have been introduced via different transmission routes. Further monitoring and research are needed to clarify its transmission dynamics and public health significance.
The MeV nucleoprotein (N protein) consists of two major domains: the structured NCORE domain (amino acids 1–400), which is responsible for viral RNA binding and nucleocapsid formation, and the intrinsically disordered NTAIL domain (amino acids 401–525), which serves as an anchor for the polymerase complex. The NTAIL domain binds to the X domain (XD) of the viral phosphoprotein, thereby inducing its folding into α-helices. Within this region, the α-helical molecular recognition element (α-MoRE) located at amino acids 488–499 plays a critical role in mediating interactions with the phosphoprotein (19). A comparison of the Gansu D8 sequences with the vaccine reference strain Shanghai-191/China-vaccine (Table 1) showed that both Clusters 1 and 2 contained 18–19 amino acid mutations. Eighteen mutations, including K405R, I406T, K441R, and Y451N, were shared by both clusters and the remaining mutations matched those in the WHO D8 reference strain. As these mutations occur outside the α-MoRE (amino acids 488–499), they are not expected to affect phosphoprotein binding mediated by this domain. Furthermore, the analysis of 150 amino acid mutation sites in the N-450 region of the Gansu D8 sequence revealed the representative sequence MVs/Gansu.CHN/23.24/7, associated with cases from Wuwei, Jiuquan, and Linxia, contained the D461G mutation in addition to the common mutations L467P, T469A, G509S, and N522D. This suggests that these cases belong to the same transmission chain. Representative sequences of MVs/Gansu.CHN/27.24/3, which has been associated with cases from Dingxi and Linxia, exhibited a G431R mutation in addition to the common mutations. These cases may form part of a distinct transmission chain. Whether the mutation sites occurring in the amino acid sequence identified in this study affect the function of the nucleoprotein requires further investigation using molecular dynamics models. Furthermore, rRT-PCR technology has a wide range of applications and is essential for MeV detection and surveillance. Three T-to-C synonymous transitions in the sequences of Cluster 1 affected the consistency of matches between primers, probes, and prevalent strains, resulting in a slight decrease in the sensitivity of the rRT-PCR assay (20). This slight decrease in sensitivity may lead to false negative results in samples with low viral loads, thereby increasing the difficulty of measles detection and surveillance. Consequently, to achieve higher resolution in the molecular surveillance of MeVs, sequencing the non-coding region between the matrix and fusion protein-coding regions or performing whole-genome sequencing may serve as complementary methods to enhance phylogenetic resolution and support transmission chain analysis beyond the standard N-450 sequencing window in future studies (15). Since the establishment of the Measles Network Laboratory in Gansu Province in 2006, all laboratory surveillance indicators have met program requirements, and the quality control of the laboratory network has been functioning effectively (11). Due to the absence of detailed epidemiological information in some cases, the origin of the D8 strains in Gansu Province remains unknown based solely on N-450 sequence comparison analysis. Further integration of epidemiological data mining with viral gene evolution analysis will aid in more accurate tracing of the transmission path of the virus, thereby providing robust molecular traceability evidence for the elimination of measles in China. Additionally, MCV timeliness and coverage decreased during the COVID-19 pandemic, and the high MeV prevalence areas in Gansu Province may be associated with delayed vaccination and inadequate coverage. This necessitates continuous efforts to strengthen vaccination education and promotion, conduct supplementary measles immunization activities, and improve vaccination management for children in migrant populations and remote impoverished areas.
| Citation: |



 Download:
Download:




