-
Introduction: On August 11, 2025, a suspected Chikungunya virus (CHIKV) case traveled from Foshan to Fuyang Xiguan Airport, Anhui Province. After symptom reporting on August 12, local CDC launched epidemiological investigations, laboratory testing, and control measures.
Methods: We collected serial blood samples throughout the patient’s hospitalization and documented the complete clinical progression from admission to discharge. Real-time fluorescent quantitative polymerase chain reaction (qPCR) was employed to detect CHIKV nucleic acid, while Enzyme-Linked Immunosorbent Assay (ELISA) was used to test for CHIKV IgG and IgM antibodies. MinION nanopore sequencing was performed on blood samples to obtain the complete viral genome sequence. Phylogenetic analyses were subsequently constructed to determine the origin, genotype, and mutation profile of this imported case.
Results: The qPCR analysis confirmed CHIKV presence in the patient’s serum samples. ELISA detected CHIKV IgG and IgM antibodies in blood samples collected on the fifth and ninth days after illness onset, respectively. Nanopore sequencing successfully generated the complete CHIKV genome sequence. Phylogenetic analysis demonstrated that the strain belonged to the East-Central-South-African lineage, consistent with the genotype identified from 190 cases in the local clustered Chikungunya fever (CHIKF) outbreak that occurred in Guangdong Province in July 2025. The strain showed 99.964% nucleotide identity (4 differential loci) with strain PX216392.1 detected in the current epidemic, and 99.9556% identity (5 differential loci) with PX236189.1.
Conclusion: This is Anhui’s first imported CHIKV case, linked to the Guangdong outbreak. No local transmission or CHIKV-positive vector mosquitoes were detected.
-
Chikungunya fever (CHIKF), caused by Chikungunya virus (CHIKV) represents a mosquito-borne arboviral disease that has emerged as a significant global public health threat (1). The clinical presentation typically manifests as an acute febrile illness accompanied by high fever, severe polyarthralgia, myalgia, rash, and headache (2). CHIKV has been classified into four distinct genotypes: East-Central-South African (ECSA), West African (WA), Asian (AL), and Indian Ocean Lineages (IOL), with the ECSA genotype demonstrating the highest virulence and most widespread global distribution (3). This represents the first detection of CHIKV in a migrant worker returning to Anhui Province, necessitating comprehensive entomological surveillance, systematic case screening, and targeted public health education in the region.
-
On August 11, 2025, a 52-year-old female patient returned to Anhui Province from Shunde District, Foshan City, Guangdong Province. Prior to departure, she had developed joint pain, fever, and scattered erythematous maculopapules on her trunk. The patient had been residing in an urban village area in Foshan characterized by high mosquito density. Despite this environmental exposure, no colleagues or family members contracted CHIKV infection, and the patient denied experiencing recent mosquito bites. She was admitted to Fuyang Second People’s Hospital on August 12.
The Fuyang CDC conducted qPCR testing on blood samples collected from both the patient and her family members. The patient’s sample tested positive for CHIKV, whereas all family members tested negative. On August 25, 2025, nucleic acid testing returned negative results (Table 1). According to the Diagnosis and Treatment Protocol for Chikungunya Fever (2025 Edition), isolation may be discontinued when body temperature normalizes for more than 24 hours with negative nucleic acid testing, or when the disease course exceeds 7 days. Following these criteria, the patient was discharged.
Time CHIKV tested by qPCR (Ct value) August 12, 2025 (+) 24.00 August 13, 2025 (+) 23.00 August 15, 2025 (+) 33.30 August 19, 2025 (+) 36.00 August 22, 2025 (+) 37.00 August 25, 2025 (–) Note: “+” indicates a positive result, defined as an amplification curve displaying a characteristic S-shape with a Ct value ≤35; "−" indicates a negative result, with Ct value >38 or no detection. Suspected positive results show a typical S-shaped amplification curve with 35< Ct value ≤38, requiring retesting. If retest results are consistent, the sample is classified as positive; if the Ct value >38 or remains undetected, the sample is classified as negative. LOD: 500 copies/mL.
Abbreviation: CHIKV=chikungunya virus; qPCR=quantitative polymerase chain reaction; LOD=limit of detection; Ct value=cycle threshold value.Table 1. Laboratory test results of blood samples from the first imported case of Chikungunya virus infection in Anhui Province.
-
The CDC performed etiological and serological testing for CHIKV, while Fuyang No.2 People’s Hospital conducted general laboratory examinations of the patient.
Upon admission, the patient exhibited elevated C-reactive protein (CRP) and serum amyloid A (SAA) levels, accompanied by abnormal liver function indicators. Following symptomatic treatment, all laboratory parameters improved and returned to normal ranges by discharge (Table 2).
Laboratory test item August 12 August 13 August 15 August 19 August 22 August 25 Reference range TP (Biuret method) (g/L) 61.7↓ 62.2↓ 63.8↓ 70.2 71 74.5 65–85 ALB (BGM) (g/L) 37.8↓ 38.6↓ 37.7↓ 41.3 42.1 45.5 40–55 GLOB (g/L) 23.9 23.6 26.1 28.9 28.9 29 20–40 A/G 1.6 1.6 1.4 1.4 1.5 1.6 1.2–2.4 PA (mg/mL) 17.9↓ 16.6↓ 18.4 23.7 27.2 30.3 18–35 ALT (U/L) 12 14 10 13 24 26 0–40 AST (U/L) 18 15 13 32 54 24 0–35 AST/ALT ratio 1.5 1.07 1.3 2.46 2.25 0.92 0.8–1.5 γ-GT (U/L) 15 13 14 27 31 20 0–45 ALP (U/L) 38↓ 45↓ 39↓ 30↓ Undetected 58 0–135 TB (μmol/L) 7.9 8.9 5.2 7.8 6.5 9.6 0–21 DB (μmol/L) 3.0 2.8 1.6 4.5 3.1 2.3 0–8.0 IB (μmol/L) 4.9 6.1 3.6 3.3 3.4 7.3 0–13 BUN (mmol/L) 6.4 4.0 4.9 4.3 4.8 4.7 2.6–7.5 Creatinine (sarcosine oxidase method) (μmol/L) 52 46 48 46 47 90 35–115 UA (μmol/L) 191 207 224 215 234 300 155–357 CRP (mg/L) 16.6↑ 16.7↑ 9.1↑ 2.5 0.7 2.1 0–6 SAA (mg/L) 277.8↑ 348.6↑ 338.1↑ 13.8↑ 3.9 Undetected 0–10 Note: ↑ means the detected value is below the upper limit of the normal reference interval; ↓ means the detected value is below the lower limit of the normal reference interval.
Abbreviation: TP=total protein; ALB(BGM)=albumin (bromocresol green method); GLOB=globulin; A/G=albumin/globulin ratio; PA=prealbumin; ALT=alanine aminotransferase; AST=aspartate aminotransferase; γ-GT=γ-glutamyl transferase; ALP=alkaline phosphatase; TB=total bilirubin; DB=direct bilirubin; IB=indirect bilirubin; BUN=blood urea nitrogen; Cr=creatinine (sarcosine oxidase method); UA=uric acid; CRP=C-reactive protein; SAA=serum amyloid A.Table 2. Serial laboratory findings during hospitalization from the first imported case of Chikungunya virus infection in Anhui Province.
CHIKV viral RNA was extracted from patient samples using a nucleic acid extraction and purification kit (Xi’an Tianlong Technology Co., Ltd., No.T183). PCR amplification was performed using a CHIKV nucleic acid detection kit employing the fluorescent PCR method [Fantasia Biopharma (Zhejiang) Co., Ltd., No.RFKNT027]. Primer sequences are presented in Table 3. Gene amplification was conducted using the SLAN 96S instrument (Shanghai Hongshi Medical Technology Co., Ltd.).
Primer/probe name Sequence (5'-3') CHIKV-F (Forward) TTTAGCCGTAATGAGCRTCGG CHIKV-PB (Probe) FAM-TGCCCACACTGTGA-MGB CHIKV-R (Reverse) CCGTGTTCGGGATCACTGTTA Abbreviations: CHIKV=chikungunya virus; PCR=polymerase chain reaction. Table 3. The nucleotide sequences of primers and probes used in the CHIKV nucleic acid PCR detection kit.
Viral amplification was performed using the CHIKV Whole Genome Capture Kit (BAIYITECH, Hangzhou, China; No. BK-CHIKV024). Amplified products underwent sequencing on a MinION Mk1B Nanopore sequencer (Oxford Nanopore Technologies, Oxford, UK). Sequencing libraries were prepared using the Sample Library Prepsystem (BJSLB-240, Hangzhou BAIYI Technology Co., Ltd., Hangzhou, China) in combination with the multiple samples DNA Library Prep Kit for Ligation Sequencing (BAIJU, Hangzhou, China; No. K024) and Ligation Sequencing Kit (ONT, UK; No. SQK-LSK110) with R9.4.1 flow cells. Data analysis was conducted using the BAIYI MicroGeno Platform (V 5.4.2, Hangzhou Baiyi Technology Co., Ltd.). Raw sequencing data underwent quality control using NanoPlot (V 1.30.07) (Coster et al., 2018), followed by filtering of low-quality reads using Filtlong (V 0.2.08). The filtered clean data were aligned to the Chikungunya reference genome using minimap2 (V 2.2210) (Li, 2018).
CHIKV IgM and IgG antibodies were detected using an Enzyme-Linked Immunosorbent Assay (ELISA) Kit (Shanghai Baipeng Biotechnology Co., Ltd., No.BP04933, No.BP04932). CHIKV IgM antibodies first appeared in the patient’s serum on the fifth day after symptom onset. IgG antibodies emerged later than IgM antibodies, and both antibody types maintained high titers throughout the subsequent monitoring period (Figure 1).
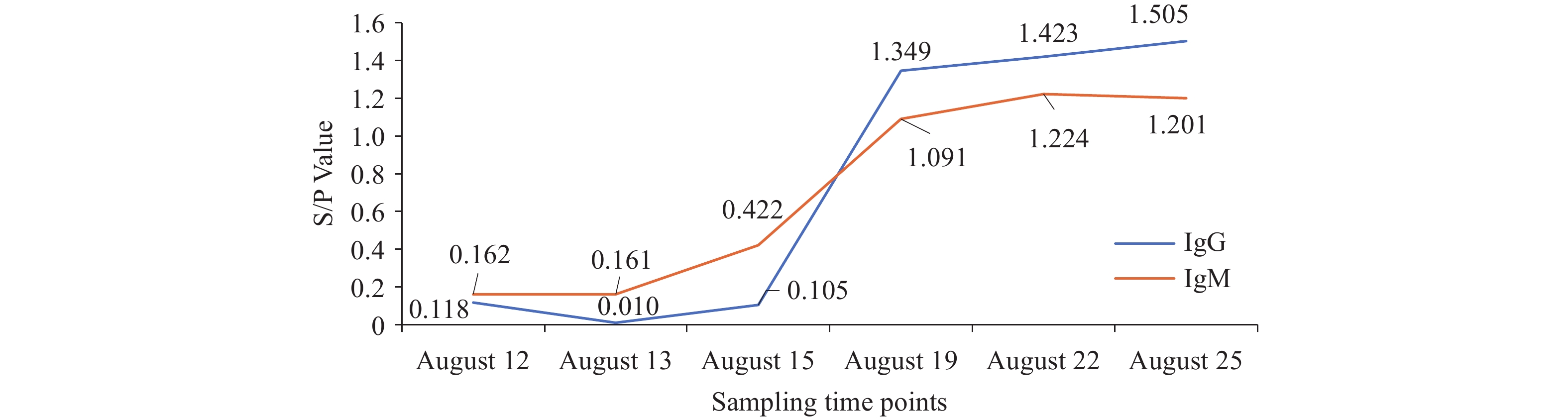 Figure 1.
Figure 1.ELISA test results for CHIKV IgG and IgM antibodies in the patient’s serum.
Note: The x-axis represents sampling time points, while the y-axis displays S/P values. Samples with S/P values ≥0.2 are classified as positive; samples with S/P values <0.2 are classified as negative. S/P=(Optical Density value of the sample to be tested−Optical Density value of the negative control)/(Optical Density value of the positive control−Optical Density value of the negative control).
Abbreviation: CHIKV=chikungunya virus; ELISA=enzyme-linked immunosorbent assay.
-
Following laboratory confirmation of CHIKV infection, the Anhui Provincial CDC, Fuyang CDC, and Yingquan CDC implemented comprehensive coordinated control measures. The patient received treatment in a mosquito-proof isolation ward at Fuyang Second People’s Hospital (also designated as Fuyang Infectious Disease Prevention and Control Hospital), while exposed individuals were monitored at home under strict mosquito prevention protocols. Using the patient’s current residence as the epicenter, three distinct surveillance zones were established: a core zone, alert zone, and monitoring zone. After case confirmation, CDCs conducted comprehensive vector surveillance in the surrounding environment and dispatched professional technical personnel to implement full-scale mosquito elimination and disinfection operations in areas adjacent to the patient’s residence. Emergency mosquito vector control measures — including rapid adult mosquito eradication, breeding site management, case isolation and treatment, and comprehensive health education — successfully contained the spread of the epidemic. As shown in Table 4, the Breteau Index (BI) was monitored daily for three consecutive days at sampling sites in core and alert zones following case confirmation. After the BI dropped below 5, surveillance was conducted twice weekly for three consecutive weeks. Following implementation of these systematic emergency mosquito vector control measures, the BI in the core zone plummeted from 83.1 to 0, while that in the alert zone also decreased to 0. No adult mosquitoes or evidence of local transmission were detected during this period.
Time Areas Household
visitsNumber of indoor and outdoor
water-holding containersNumber of positive
containersBI August 13, 2025 Core zones 71 235 59 83.1 Alert zones 69 178 42 60.9 Monitoring zones − − − − August 14, 2025 Core zones 72 16 7 9.72 Alert zones 95 83 8 8.42 Monitoring zones − − − − August 15, 2025 Core zones 76 8 3 3.95 Alert zones 101 37 4 3.96 Monitoring zones − − − − August 20, 2025 Core zones 93 15 0 0 Alert zones − − − − Monitoring zones − − − − August 22, 2025 Core zones 105 3 0 0 Alert zones 103 13 0 0 Monitoring zones − − − − August 26, 2025 Core zones 109 14 0 0 Alert zones − − − − Monitoring zones 61 17 1 1.64 August 29, 2025 Core zones 106 11 0 0 Alert zones 99 5 0 0 Monitoring zones − − − − September 1, 2025 Core zones 104 10 0 0 Alert zones 96 8 0 0 Monitoring zones 56 6 0 0 Note: The BI survey represents a standardized methodology for rapidly assessing Aedes mosquito density, the primary vector for CHIKV transmission. “-” means undetected.Using BI values as the primary indicator for evaluating Aedes mosquito density, the classification system encompasses four distinct grades: high density (BI>20), medium density (10<BI≤20), low density (5<BI≤10), and acceptable control levels meeting prevention requirements (BI≤5).
Abbreviation: BI=breteau index; CHIKV=chikungunya virus.Table 4. Chikungunya fever emergency mosquito surveillance data.
-
The global epidemiology of CHIKV has evolved dramatically in recent decades. Originally confined to tropical and subtropical regions, CHIKV has expanded into temperate countries due to global warming and increased human mobility. Most patients develop fever, joint pain, and rash, while some experience neurological complications or death. On October 3, 2025, the World Health Organization (WHO) issued a critical warning, noting that CHIKV has been detected in 119 countries and regions worldwide. From January to September 2025, 263,592 suspected and 181,679 confirmed CHIKV disease cases and 155 CHIKV disease-related deaths have been reported globally, making epidemic control increasingly challenging worldwide (4).
In July 2025, an imported CHIKV outbreak emerged in Shunde District, Foshan City, Guangdong Province, China (5). Guangdong subsequently experienced China’s largest locally clustered outbreak originating from imported cases. By September 13, cumulative confirmed cases reached 10,873, demonstrating CHIKV’s significant local transmission potential in China (6). On August 11, 2025, Fuyang CDC identified a suspected case in an individual returning from Shunde District. Laboratory testing confirmed CHIKV infection, marking the first imported CHIKF case in Anhui Province. Phylogenetic analysis confirmed that the strain belonged to the East/Central/South African (ECSA) genotype (Figure 2).
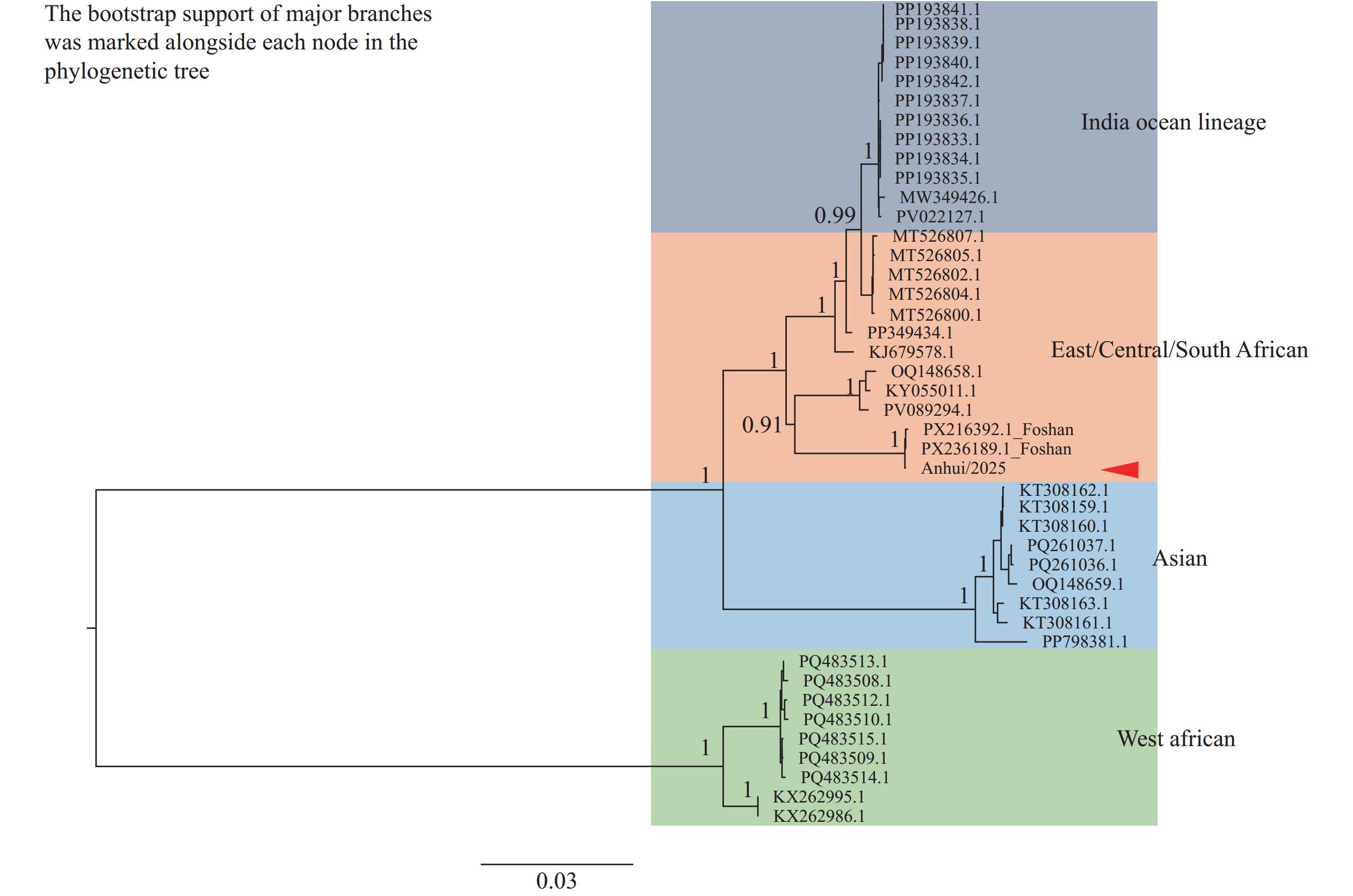 Figure 2.
Figure 2.Phylogenetic analysis of Anhui|China|August-2025 CHIKV whole-genome sequences using the neighbor-joining method.
Note: Anhui2025 represents the viral strain isolated from the first imported CHIKV case detected in Anhui Province. Abbreviation: CHIKV=chikungunya virus.Epidemiological investigation and molecular tracing confirmed this case as an imported infection acquired in Foshan City, with symptom development occurring before the patient’s return to Anhui Province. The 52-year-old female patient developed symptoms on August 11 and returned from Shunde District — the CHIKV outbreak epicenter in Guangdong Province — on the same day. On August 12, the patient’s family contacted the local CDC to report her residence history in the epidemic area. The Fuyang CDC responded immediately by arranging urgent treatment and organizing comprehensive epidemiological investigations, pathogen testing, environmental monitoring, and surveillance activities. Through timely intervention, this imported case did not result in local transmission. Although two vaccines have been approved (with IXCHIQ suspended by the U.S. Food and Drug Administration on August 22, 2025), no vaccine has achieved widespread global implementation to date. Personal mosquito prevention therefore remains the primary strategy for CHIKF prevention in most areas (7). Following CDC prevention and control measures, virus transmission risk in both core epidemic and warning zones decreased to safe levels (indicators below 5) within 3 days (Table 4). The confirmation and isolation of the patient within 2 days of symptom onset underscores that collective vigilance at the family and individual levels is essential for achieving early detection, diagnosis, and treatment of imported cases. Zhao Jin et al. (8) similarly emphasized that early diagnosis and evidence-based prevention measures are crucial for effective CHIKF control.
The patient exhibited characteristic CHIKF manifestations: fever, rash, and joint pain. Joint pain emerged first at onset, primarily affecting the interphalangeal joints of both hands and knee joints, followed by fever. Scattered erythematous maculopapules appeared on the extremities without obvious pruritus, resolving 10 days after symptom onset. These clinical features align with CHIKF symptoms reported in both domestic and international literature (9-10).
Comparison with an imported CHIKF case detected in Haikou City, Hainan Province on July 28, 2025, revealed that both patients presented with mild symptoms and were confirmed by CHIKV nucleic acid testing in the early stage of illness (11). Nevertheless, the case in this study exhibited the classic CHIKF triad symptoms at an early stage, whereas the Haikou case first developed a rash and fever without joint pain, followed by myalgia on the third day after onset. Regarding the genotypic distribution of CHIKV detected in China (12), the epidemic strains are predominantly of the ECSA genotype, followed by the Asian genotype — our sequencing results are consistent with this epidemic pattern.
Clinical laboratory results obtained on the admission day (second day after symptom onset) revealed elevated CRP and SAA levels (Table 5), indicating an acute inflammatory response. This finding aligns with the viremia and systemic inflammation characteristic of CHIKV infection, representing typical laboratory manifestations during the acute phase (13). Among liver and kidney function indicators, total protein (biuret method), albumin (bromocresol green method), prealbumin, and alkaline phosphatase levels fell below normal ranges (14). This pattern suggests that CHIKV infection may induce hepatocellular damage, warranting further investigation. The patient’s hematological profile demonstrated increased neutrophil percentage alongside decreased lymphocyte and eosinophil percentages, with reduced absolute lymphocyte and eosinophil counts. White blood cell and platelet counts remained within normal limits (Table 5). These characteristics align with hematological findings reported in previous CHIKV outbreaks (13–15). Comparison of nucleic acid and antibody test results revealed that during early CHIKV infection, RNA detection provides superior sensitivity compared to antibody detection. However, as the disease progresses, viral RNA disappears rapidly while serum antibodies gradually emerge and persist long-term (16–19). Real-time PCR represents a more reliable method for detecting acute-phase cases, whereas antibody detection better suits determining previous viral exposure. The selection of CHIKV detection methods should be determined based on research objectives and sample collection timing. These laboratory indicators comprehensively confirm the patient’s acute CHIKV infection across four dimensions: viral presence (positive nucleic acid), inflammatory response (elevated inflammatory markers), organ function impact (reduced liver-related proteins), and immune cell changes (abnormal complete blood count). They provide multiple lines of evidence for clinical diagnosis and serve as reference indicators for formulating treatment plans and monitoring patient condition.
Laboratory test item August 13 Results tips Reference range TP (Biuret method) (g/L) 62.2 ↓ 65–85 ALB (BCG) (g/L) 38.6 ↓ 40–55 GLOB (g/L) 23.6 20–40 A/G 1.6 1.2–2.4 PA (mg/mL) 16.6 ↓ 18–35 ALT (U/L) 14 0–40 AST (U/L) 15 0–35 AST/ALT Ratio 1.07 0.8–1.5 γ-GT (U/L) 13 0–45 ALP (U/L) 45 ↓ 50–135 TB (μmol/L) 8.9 0–21 DB (μmol/L) 2.8 0–8.0 IB (μmol/L) 6.1 0–13 BUN (μmol/L) 4 2.6–7.5 Creatinine (Sarcosine Oxidase Method) (μmol/L) 46 35–115 UA (μmol/L) 207 155–357 ECC (mL/min) 152.61 ↑ 80–120 Glu (mmol/L) 4.16 3.89–6.11 K (mmol/L) 3.39 ↓ 3.5–5.3 Na (mmol/L) 137.8 137–147 Cl (mmol/L) 104.2 99–110 Ca (mmol/L) 2.09 2.03–2.67 CRP (mg/L) 16.7 ↑ 0–6 SAA (mg/L) 348 ↑ 0–10 WBC (×109/L) 3.68 3.5–9.5 NP (%) 80.4 ↑ 40–75 LP (%) 12.8 ↓ 20–50 MP (%) 6.4 3–10 EP (%) 0.1 ↓ 0.4–8.0 BP (%) 0.3 0–1 ANC (×109/L) 2.96 1.8–6.3 ALC (×109/L) 0.47 ↓ 1.1–3.2 AMC (×109/L) 0.24 0.1–0.6 AEC (×109/L) 0.00 ↓ 0.02–0.52 ABC (×109/L) 0.01 0–0.06 RBC (×1012/L) 4 3.8–5.1 Hb (g/L) 117 115–150 Hct (%) 35.8 35–45 MCV (fL) 90.4 82–100 MCH (pg) 29.6 27–34 MCHC (g/L) 327 316–354 RDW-CV (%) 12.8 11.6–16 PLT (×109/L) 137 125–350 MPV (fL) 11.4 6–13 PDW (%) 16.3 9–18 P-LCR (%) 36.2 17.5–42.3 PCT (%) 0.157 0.114–0.282 Note: “↓” means the detected value is above the lower limit of the normal reference interval;“↑”means the detected value exceeds the upper limit of the normal reference interval.
Abbreviation: TP=total protein; ALB=albumin; GLOB=globulin; A/G=albumin/globulin ratio; PA=prealbumin; ALT=alanine aminotransferase; AST=aspartate aminotransferase; γ-GT=γ-glutamyl transferase; ALP=alkaline phosphatase; TB=total bilirubin; DB=direct bilirubin; IB=indirect bilirubin; BUN=blood urea nitrogen; UA=uric acid; ECC=endogenous creatinine clearance; Glu=glucose; K=potassium; Na=sodium; Cl=chloride; Ca=calcium; CRP=C-reactive protein; SAA=serum amyloid A; WBC=white blood cell; NP=neutrophil percentage; LP=lymphocyte percentage; MP=monocyte percentage; EP=eosinophil percentage; BP=basophil percentage; ANC=absolute neutrophil count; ALC=absolute lymphocyte count; AMC=absolute monocyte count; AEC=absolute eosinophil count; ABC=absolute basophil count; RBC=red blood cell; Hb=hemoglobin; Hct=hematocrit; MCV=mean corpuscular volume; MCH=mean corpuscular hemoglobin; MCHC=mean corpuscular hemoglobin concentration; RDW-CV=red blood cell distribution width-coefficient of variation; PLT=platelet count; MPV=mean platelet volume; PDW=platelet distribution width; P-LCR=platelet large platelet ratio; PCT=platelet crit.Table 5. Baseline Laboratory Results on Admission from the first imported case of Chikungunya virus infection in Anhui Province.
The patient demonstrated mild symptoms and achieved favorable outcomes with symptomatic treatment. However, this single-case report has inherent limitations. The sample size precludes generalization regarding disease characteristics such as incidence rates, clinical manifestation diversity, and prognostic variability across the broader patient population. Furthermore, the absence of a control group prevents conclusions regarding clinical variation or transmission efficiency of chikungunya fever among local vector populations in Anhui Province. Future studies incorporating larger case series will be necessary to validate these preliminary findings and establish more robust epidemiological patterns.
This first imported CHIKF case in Anhui Province highlights China’s increasing vulnerability to imported infections and the growing risk of local transmission. The successful prevention of secondary transmission can be attributed to two critical factors: prompt patient reporting and a robust regional surveillance system equipped with advanced diagnostic capabilities. Together, these elements enabled the “early detection, rapid response, and immediate isolation” framework essential for effective disease control.
This incident demonstrates the urgent need to strengthen infectious disease surveillance systems targeting returning migrant workers. Effective prevention and control strategies should focus on three core areas: source control, transmission interruption, and protection of vulnerable populations. Interdepartmental coordination between port health authorities, local CDCs, and hospitals requires enhancement through several mechanisms. For instance, health declarations and vector screening should be strengthened for individuals arriving from affected areas at entry ports. Mandatory CHIKV screening should be implemented for travelers from WHO-designated high-risk areas, and joint vector control drills should be conducted within 24 hours of case reporting. These findings provide valuable guidance for managing similar imported infectious disease threats throughout China.
-
Approved by The Ethics Committee of Anhui Center for Disease Control and Prevention, China (approval number SL2024-73003-01).
HTML
Epidemiological Investigation
Laboratory Test of the Patient
| Citation: |



 Download:
Download:




