-
Introduction: To evaluate population immunity against the mumps virus (MuV) after China’s two-dose Measles-Mumps-Rubella (MMR) vaccine policy was introduced in June 2020, we conducted a sero-epidemiological analysis among individuals aged 0–19 years.
Methods: A cross-sectional survey was conducted in Fujian Province from March to June 2023. MuV IgG antibodies were detected using ELISA, and their seroprevalence and geometric mean concentrations (GMCs) were assessed. Population immunity in 2023 was compared with a 2018 survey.
Results: Overall seroprevalence and GMCs in 2023 were 79.53% and 265.61 U/mL, respectively. Both varied significantly by age: levels rose from 8 months, peaked at 2–5 years, then declined to a low point at 15–17 years. In 2023, seroprevalence and GMCs were higher in children aged 0–1 year (47.29% vs. 14.51%; 97.15 U/mL vs. 34.13 U/mL) and lower in those 15–17 years (58.06% vs. 80.73%; 126.00 U/mL vs. 289.98 U/mL) compared with 2018; 2-year-olds showed higher GMCs (554.85 U/mL vs. 353.39 U/mL). Among vaccinated individuals, antibody levels peaked 15–90 days after the latest vaccination; two-dose recipients exhibited significantly higher antibody levels than one-dose recipients within 270 days.
Conclusions: China’s two-dose MMR vaccine policy has effectively increased seroprevalence and GMCs in children 8 months-2 years. Continuous monitoring of antibody decline is essential, particularly for those aged 6–19 years with weaker immunity.
-
Mumps, an acute infectious disease caused by the mumps virus (MuV), is clinically characterized by fever and swelling of the parotid glands and can lead to severe complications, including meningitis, orchitis, sensorineural deafness, myocarditis, pancreatitis, and even death. Although it primarily affects pediatric populations, surveillance data indicate a substantial disease burden among post-pubertal individuals (1). In the absence of specific antiviral therapies, vaccination remains the cornerstone of mumps prevention. Since 2008, a single dose of the trivalent live attenuated measles-mumps-rubella (MMR) vaccine has been administered at 18 months of age as part of China’s national immunization program. However, national surveillance data from 2004 to 2021 show persistent mumps transmission despite vaccination efforts (2). Provincial-level surveillance in Fujian Province from 2014 to 2019 documented approximately 3,200 annual cases, with a bimodal age distribution peaking at 4–6 years and 9–15 years, suggesting suboptimal vaccine effectiveness in school-aged populations (3). Accumulating evidence indicates that a single MMR dose provides only limited long-term protection due to time-dependent waning immunity (4–5). To address this issue, China’s National Immunization Program introduced a two-dose MMR policy in 2020, replacing the measles-rubella (MR) vaccine with the MMR vaccine at 8 months, followed by a booster dose at 18 months. From 2020 to 2022, the number of reported mumps cases in Fujian Province showed a continuous decline, with only 1,512 cases reported in 2022 (6). This seroepidemiological study aims to systematically assess population immunity to the mumps virus in 2023 and compare it with baseline data from 2018. These findings will provide actionable insights to guide the optimization of MMR vaccination strategies and support efforts to achieve mumps elimination targets.
In 2023, a cross-sectional serological survey was conducted among individuals aged 0–19 years in Fujian Province, China. Ten counties from ten cities were randomly selected, and two villages or communities were randomly chosen from each county. The minimum sample size required for the survey was calculated independently for each city, resulting in a value of N=263. The sample size was determined using the formula: N=Zα2p(1-p)/d2, where the parameters were defined as follows: an expected overall mumps seropositivity rate (p) of 0.78 (3), a significance level (α) of 0.05 (Zα=1.96), and an allowable margin of error (d) of 0.05. A systematic sampling method was used to select individuals from each village or community, stratified by age and gender. Individuals with fever, chronic infectious diseases, or immunosuppression were excluded. Each participant provided 2–3 mL of venous blood and completed a questionnaire on personal information and immunization history. The study was approved by the Fujian Provincial Center for Disease Control and Prevention [Approval No: Fujian Provincial Center for Disease Control and Prevention Ethical Review Approval (2021) No. (016)].
MuV IgG antibodies were detected using commercial mumps virus IgG ELISA kits (SERION ELISA classic, Institute Virion/Serion GmbH, batch number: EO0014). According to the manufacturer’s instructions, samples with concentrations ≥100 U/mL were considered positive, while those with concentrations <100 U/mL were considered negative.
To compare the temporal dynamics of antibody levels following vaccination under two distinct MMR vaccination strategies, we conducted a retrospective cohort study. The study population was drawn from the 2023 and 2018 surveys. The two-dose MMR group included children who received two doses of the MMR vaccine at 8 months and 18 months (2023 survey). The one-dose MMR group consisted of age-matched children who had received only a single MMR dose at 18 months (2018 survey). The 2018 survey was a population-based cross-sectional serosurvey conducted in Fujian Province in 2018 and included 4,925 participants aged 0–60 years (3).
Data from the questionnaires were entered into EpiData 3.1 software. Seroprevalence, with corresponding 95% confidence intervals (95% CI), and the GMCs of MuV IgG antibodies were calculated. Differences in seroprevalence and GMCs were assessed using the Chi-square test, Student’s t-test, analysis of variance (ANOVA), and Pearson’s correlation analysis, as appropriate. Statistical analyses were performed using SPSS Statistics 24.0 and GraphPad Prism 8.0.1, with two-sided P-values <0.05 considered statistically significant.
A total of 2,711 subjects aged 0–19 years were recruited. The overall seroprevalence was 79.53% (95% CI: 78.01%–81.05%), and the GMC was 265.61 U/mL (95% CI: 253.79–278.55 U/mL). Both seroprevalence and GMCs differed significantly across age groups and vaccination statuses. Among individuals aged 0–19 years, seroprevalence ranged from 22.06% to 96.10%, and GMCs ranged from 53.88 U/mL to 554.85 U/mL (Table 1). The lowest seroprevalence was observed in the 0–7-month age group (0≤ to <8 months), peaking among those aged 2–5 years and then gradually declining in the 15–17-year age group (15≤ to <18 years). Seroprevalence among individuals aged 2–17 years showed a significant downward trend (χ2=268.30, P<0.001). GMCs were 53.88 U/mL at 0–7 months, rose sharply to 263.09 U/mL between 8 months and 1 year, and peaked at 554.85 U/mL at age 2. Thereafter, GMCs gradually declined to a low of 126.00 U/mL over the next 15 years. Notably, GMCs among individuals aged 2–17 years demonstrated a negative correlation with age by Pearson correlation analysis (r=−0.47, P<0.001).
Characteristic Sample No. Seroprevalence % (95% CI) χ2, P GMCs (95% CI) (U/mL) t/F,P Gender Male 1,475 79.53 (77.46, 81.59) 0.00,
1.000261.83 (247.03, 278.99) 0.664,
0.507Female 1,236 79.53 (77.28, 81.78) 270.19 (252.17, 289.93) Age 0≤ to <8 months 68 22.06 (11.95, 32.17) 480.99,
<0.00153.88 (41.54, 70.26) 98.54,
<0.0018≤ to <24 months 479 76.00 (72.15, 79.83) 263.09 (228.11, 302.75) 2≤ to <3 years 270 95.19 (92.62, 97.76) 554.85 (495.81, 616.27) 3≤ to <4 years 263 93.92 (91.01, 96.82) 419.99 (378.88, 464.68) 4≤ to <5 years 287 94.08 (91.33, 96.82) 442.89 (399.13, 489.03) 5≤ to <6 years 282 96.10 (93.83, 98.37) 425.59 (385.74, 464.15) 6≤ to <12 years 267 85.02 (80.71, 89.33) 289.94 (258.78, 324.09) 12≤ to <15 years 241 70.12 (64.30, 75.94) 150.10 (133.47, 169.88) 15≤ to <18 years 279 58.06 (52.24, 63.89) 126.00 (110.80, 144.01) 18≤ to <20 years 275 63.27 (57.54, 69.01) 146.39 (128.10, 166.20) Total 2,711 79.53 (78.01, 81.05) 265.61 (253.79, 278.55) Vaccination status 516.26,
<0.001305.60,
<0.0010 dose 208 31.25 (24.9, 37.60) 58.84 (49.01, 70.51) 1 dose 1,283 83.63 (81.60, 85.66) 270.66 (256.39, 286.74) ≥2 dose 753 96.15 (94.77, 97.53) 567.22 (528.34, 600.82) Unknown 237 59.92 (53.63, 66.20) 136.61 (118.03, 159.75) Total 2,481 80.77 (79.22, 82.33) 279.27 (265.87, 292.17) Abbreviation: GMC=geometric mean concentration; CI=confidence intervals.
Note: Seroprevalence among different groups was compared using the chi-square test. For GMCs, comparisons between two groups were analyzed using the t-test, and comparisons among three or more groups were assessed using analysis of variance (ANOVA).Table 1. Seroprevalence and GMCs of MuV IgG by gender, age, and vaccination status among individuals among aged 0–19 years in Fujian Province in 2023.
A total of 2,481 subjects completed questionnaires regarding their mumps-containing vaccine history. Based on vaccination status, the distribution of individuals receiving 0 doses, 1 dose, ≥2 doses, and unknown status was 8.38%, 51.71%, 30.35%, and 9.55%, respectively. Seroprevalence and GMCs in vaccinated children were significantly higher than in unvaccinated children. Furthermore, children who received ≥2 doses of the vaccine exhibited higher seroprevalence and GMCs than those who received only 1 dose.
Due to differing age distributions between the two studies, we calculated standardized seroprevalence estimates of MuV IgG for 2023 and 2018. The seroprevalence and GMCs in the 2023 study population (79.53%, 265.61 U/mL) were significantly higher than those in the 2018 population (74.65%, 229.06 U/mL) (χ2=20.09, P<0.001; t=4.47, P<0.001). In the 0–1-year age group, seroprevalence and GMCs in 2018 (14.51%, 34.13 U/mL) were significantly lower than those in 2023 (47.29%, 97.15 U/mL) (χ2=74.90, P<0.001; t=9.314, P<0.001). Both surveys showed high seroprevalence in the 2–5-year age group, followed by a gradual decline beginning at age 6. A notable decrease was observed in adolescents aged 15–17, with seroprevalence and GMCs in 2023 significantly lower than those in the corresponding age group in 2018 (80.73%, 289.98 U/mL) (χ2=37.053, P<0.001; t=8.978, P<0.001). GMCs for 2-year-olds in 2023 were also significantly higher than those for the same age group in 2018 (353.39 U/mL) (t=5.916, P<0.001). Both studies demonstrated a declining trend in GMCs over time, with a notable widening of the disparity between the two cohorts after age 12, as markedly lower GMCs were recorded in 2023 compared with 2018 (Figure 1).
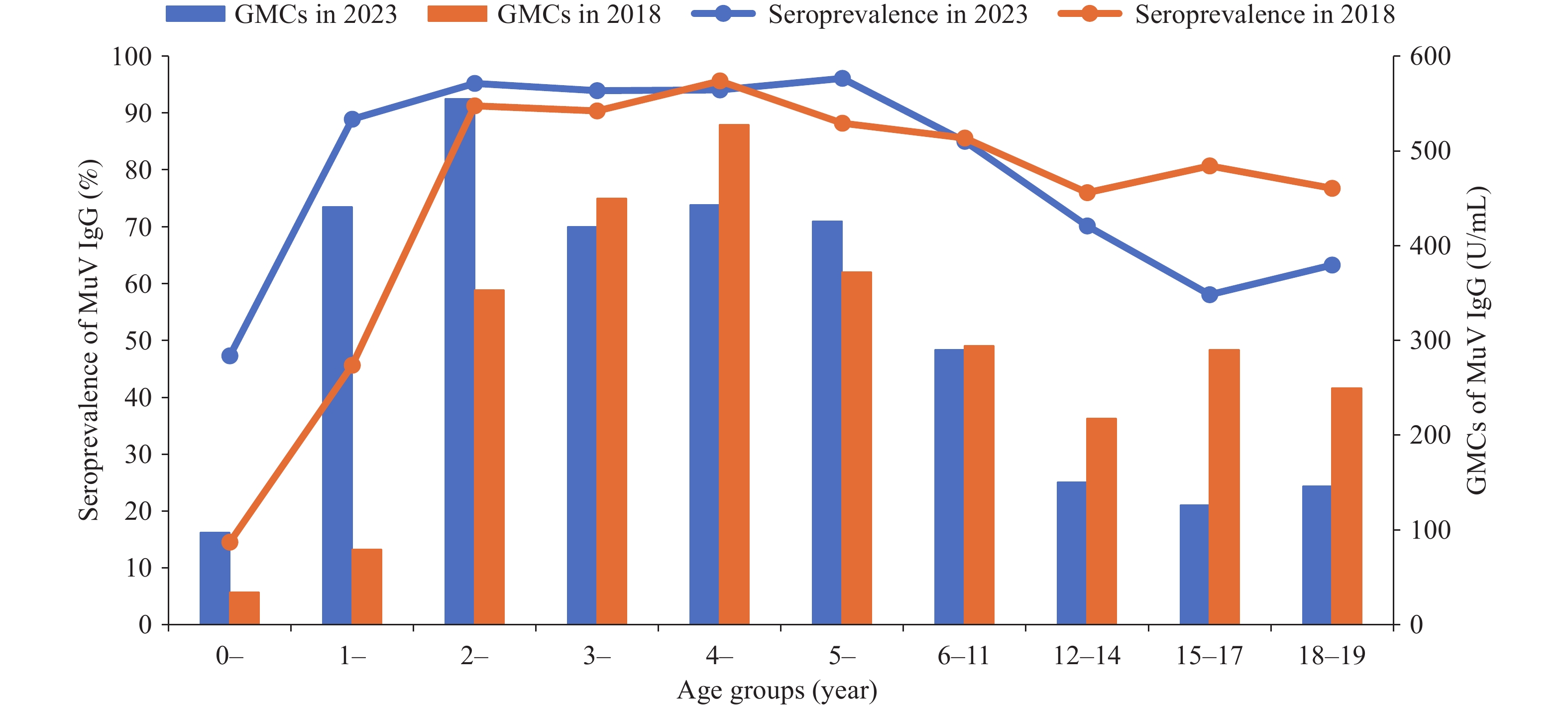 Figure 1.
Figure 1.Seroprevalence and GMCs of MuV IgG in 2023 and 2018.
Note: The figure shows mumps antibody levels across various age groups in 2023 and 2018. Blue bars represent the GMCs of mumps antibodies in 2023, while orange bars represent the corresponding GMCs in 2018. The blue line indicates the seropositivity rate of mumps antibodies in 2023, and the orange line shows the seropositivity rate in 2018.
Abbreviation: GMC=geometric mean concentration.
In the 2023 survey, we identified 329 participants (male: 57.14%; female: 42.86%) aged 1.5–4 years (median: 2.45 years) who had received two doses of the MMR vaccine at 8 months and 18 months, respectively. The interval since the second dose ranged from 0 to 1,079 days (median: 303 days). Additionally, we identified 557 participants (male: 56.91%; female: 43.09%) aged 1.5–4 years (median: 2.70 years) from the 2018 survey who had received a single MMR dose at 18 months. The interval since vaccination in this group ranged from 0 to 885 days (median: 385 days). Based on the time since vaccination, participants were categorized into eight intervals: 0–14, 15–90, 91–180, 181–270, 271–360, 361–450, 451–540, 541–630, 631–720, and 721–1,079 days post-vaccination.
Among participants who received two MMR doses, GMCs were 204.12 U/mL within 0–14 days post-vaccination, peaked at 1468.12 U/mL between 15 and 90 days, and then declined to 516.39 U/mL at 271–360 days. Thereafter, GMCs remained relatively stable, fluctuating between 502.69 U/mL and 693.80 U/mL. A similar pattern was observed in participants who received one MMR dose, with GMCs increasing from 32.74 U/mL at 0–14 days to 434.89 U/mL at 15–90 days, followed by a steady plateau. Antibody levels in two-dose recipients within 270 days of the last vaccination were significantly higher than those in one-dose recipients (P<0.001). However, this difference gradually diminished, with levels approaching similarity by 271–360 days post-vaccination (Figure 2).
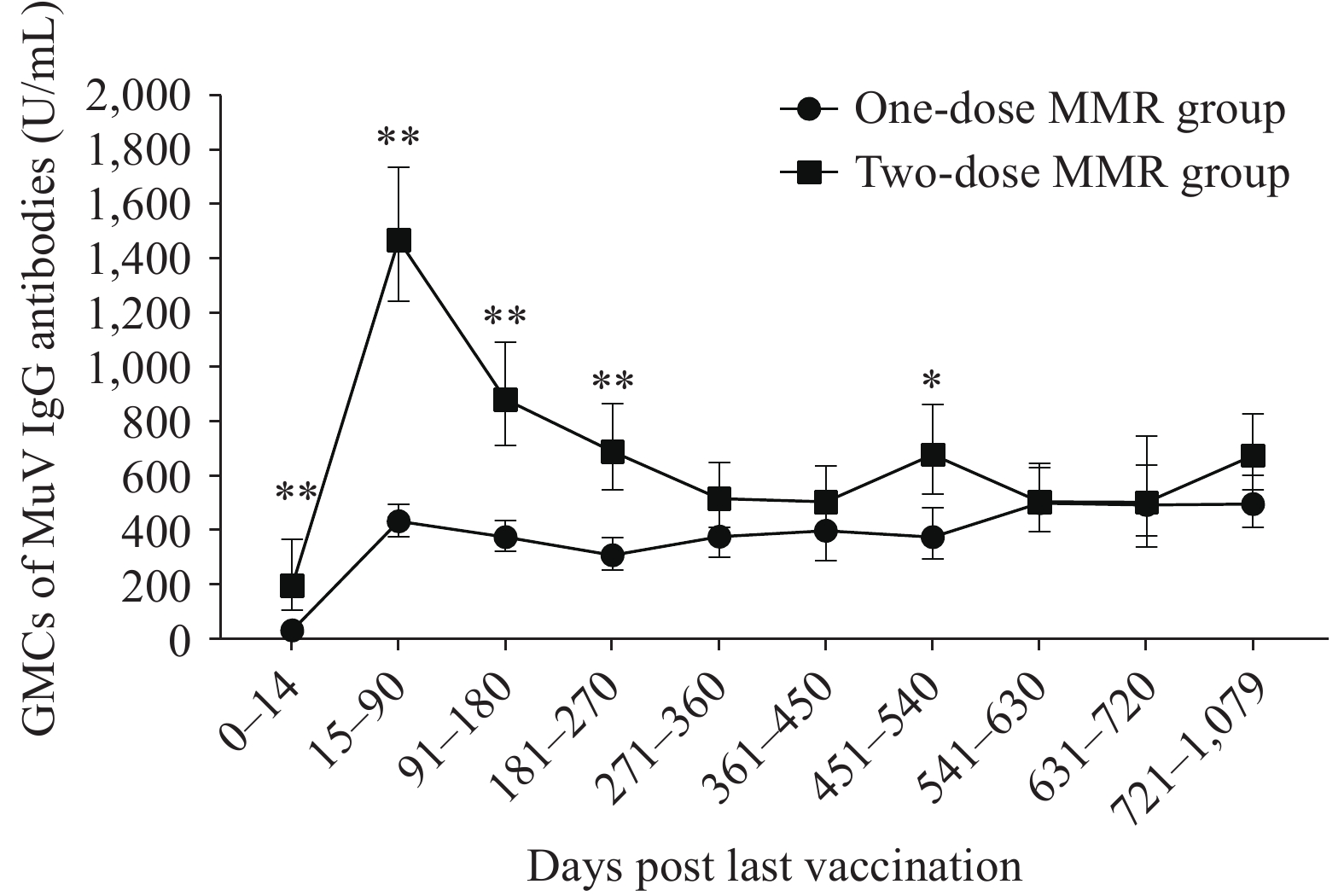 Figure 2.
Figure 2.Dynamic changes in MuV IgG antibody levels following vaccination with different doses of the MMR vaccine.
Note: The figure presents the GMCs of MuV IgG antibodies in populations that received two different MMR vaccination regimens, measured at multiple time points after vaccination. The dotted line represents the temporal changes in antibody levels among individuals in the one-dose MMR group, while the square-marked line shows the corresponding dynamics in the two-dose MMR group.
Statistical significance between the two groups is indicated by asterisks: *P<0.01 and **P<0.001.
Abbreviation: GMC=geometric mean concentration; MMR=measles-mumps-rubella.
-
This study examined mumps virus antibody levels in children aged 0–19 years in Fujian Province, three years after the implementation of the two-dose MMR vaccine policy in 2020. Compared with data from 2018, overall antibody levels showed a slight increase in 2023, with the most substantial improvement observed in children aged 8 months-2 years. In this age group, both seroprevalence (rising from 76.00% to 95.19%) and GMCs (increasing from 263.09 to 554.85 U/mL) improved markedly. Children aged 3–5 years maintained high antibody levels, with seroprevalence ranging from 93.92% to 96.10% and GMCs between 419.99 and 442.89 U/mL. However, antibody levels began to decline gradually from 6 years of age onward. In 2-year-olds, antibody levels induced by two MMR doses were significantly higher than those generated by a single dose. Nevertheless, within 360 days after the second dose, antibody levels gradually decreased to levels comparable to those in children who had received only one dose (MMR1).
Previous studies have reported that the immunological boost in MuV antibodies following a second (MMR2) or third (MMR3) dose of the MMR vaccine is temporary, with antibody levels returning to pre-boost levels within approximately one year (7–8). Our study confirms this finding: MuV antibody levels initially rose in response to MMR2 but declined to near MMR1 levels within one year. The transient nature of this boost may be attributed to the low frequency of mumps-specific memory B cells generated after MMR vaccination, which fails to establish strong long-term B-cell memory (9). Despite the post-MMR2 decline in antibody levels, children aged 3–5 years still maintained sufficient levels to support high herd immunity against mumps.
It is noteworthy that there has been a continuous decline in antibody levels among individuals aged 6–19 years who received only one dose of the MMR vaccine, placing them in a high-risk window for mumps infection. Their seroprevalence ranged from 58.06% to 85.02%, significantly lower than the estimated herd immunity threshold of 88%–92% (10). Children aged 15–17 years, the first cohort to receive the MMR vaccine under the Expanded Program on Immunization (EPI), have become a particularly vulnerable population in 2023, as reflected by a relatively low seroprevalence of 58.06% and antibody titers of 126 U/mL. In contrast, their seroprevalence and GMCs in 2018 were 80.73% and 289.98 U/mL, respectively, indicating a marked decline. Furthermore, high contact rates and population density in primary and senior high schools may facilitate mumps transmission, potentially overcoming vaccine-induced protection among students (11). Notably, most outbreaks in primary and junior high schools have occurred in populations with high vaccination coverage (12–13). Between 2017 and 2021, 72.92% of mumps cases in individuals under 18 years old were breakthrough infections, with this proportion rising to 90.78% in 2021 in Huzhou City, China (14). Numerous studies have confirmed that students who received their last dose of a mumps-containing vaccine (MuCV) more than 10 years before an outbreak face an increased risk of contracting mumps (12,15).
The study has certain limitations. First, it was conducted only three years after the implementation of the two-dose MMR vaccination program, which limits our ability to capture long-term serological dynamics in vaccinated populations. Second, although a cohort study would be optimal for tracking antibody kinetics across multiple time points post-vaccination, this research used a cross-sectional design, which is primarily descriptive and serves as an early warning signal. Additionally, cross-sectional studies cannot distinguish antibody increases caused by natural infection after vaccination, which may lead to an overestimation of vaccine-induced protection.
In conclusion, the implementation of the two-dose MMR vaccination policy has resulted in high seroprevalence and GMCs among children aged 8 months to 2 years. However, continuous monitoring of antibody waning is essential. Enhanced surveillance of mumps in children and adolescents aged 6–19 years is also critical due to their relatively low antibody levels.
-
All the unnamed participants in this study, particularly the CDC staff at all levels who were involved in the serosurvey.
HTML
| Citation: |



 Download:
Download:




