-
Introduction: On September 11, 2024, a school in the Beijing Economic and Technological Development Area (Jingkai area) reported multiple cases of fever. Public health professionals from the local CDC promptly initiated an on-site epidemiological investigation.
Methods: We conducted an initial epidemiological assessment and rapidly identified the index case. Simultaneously, we performed active case finding throughout the school. Throat swab samples were collected from symptomatic individuals and submitted to the laboratory for pathogen testing. Real-time RT-PCR was used to screen for a comprehensive panel of respiratory pathogens. We established viral cultures and performed PCR to amplify target gene sequences. Molecular characterization was conducted using gene homology analysis and phylogenetic reconstruction.
Results: Active case finding identified 15 fever cases (37.8 °C–39.9 °C), all from two adjacent classes in the school. Case onset was concentrated between September 9 and 11, with 6 males and 9 females, all aged 6 years. Fourteen throat swab samples were collected, with 10 testing positive for HAdV genes. Six viral strains were successfully isolated through cell culture. Sequence analysis revealed 100% gene homology in the hexon Loop2 region, consistent with HAdV-B3. Furthermore, the penton base gene, hexon gene, and fiber gene of all 6 strains exhibited 100% homology. The penton base gene showed 99.2% homology with the HAdV-B7 reference strain, while the hexon and fiber genes demonstrated 99% and 96.8% homology, respectively, with the HAdV-B3 reference strain. The genotype of all 6 strains was P7H3F3, consistent with the HAdV-B114 (P7H3F3) genotype, confirming their identification as HAdV-B114.
Conclusions: This outbreak was caused by a novel recombinant human adenovirus, HAdV-B114. Given the potential for such emerging HAdV strains to trigger infectious disease outbreaks, enhanced surveillance systems and comprehensive molecular characterization are essential for early detection and effective public health response.
-
Human adenovirus (HAdV) represents a significant viral pathogen capable of infecting multiple vital organ systems, including the respiratory tract, ocular tissues, and gastrointestinal tract (1). HAdV belongs to the genus Mastadenovirus within the family Adenoviridae and is characterized as a non-enveloped virus containing linear double-stranded DNA. The viral genome spans approximately 36 kb and encodes roughly 40 proteins, among which the most critical structural components are the penton base, hexon, and fiber proteins (1-2). The penton base protein facilitates viral endocytosis and promotes cellular infection (1-2). The hexon protein contains two highly variable loop domains, Loop1 and Loop2, which are associated with serotype-specific antigenic differences and host immune responses, making them valuable tools for preliminary HAdV strain identification (1-2). The fiber protein mediates viral attachment by binding to specific host-cell receptors.
HAdV classification is based on biological characteristics and genetic homology, resulting in seven distinct subgenera (A–G). The genes encoding the penton base, hexon, and fiber proteins are particularly susceptible to homologous recombination events. According to the Human Adenovirus Working Group guidelines, accurate genotyping requires analysis of the complete sequences of these three critical genes (http://hadvwg.gmu.edu).
In September 2024, an acute respiratory outbreak occurred at an elementary school in the Beijing Economic and Technological Development Area. Local CDC professionals responded by conducting comprehensive epidemiological investigations and collecting throat swab samples from affected individuals. Laboratory analysis confirmed adenoviral infection, prompting immediate implementation of prevention and control measures. Subsequent molecular characterization and phylogenetic analysis revealed that the outbreak was caused by a novel recombinant HAdV-B114, which emerged through genetic recombination between HAdV-B3 and HAdV-B7.
-
On September 11, 2024, two classes at an elementary school in the Jingkai area reported multiple fever cases. Public health professionals from the local CDC immediately conducted an on-site epidemiological investigation. The school housed 189 classes with 7,368 students and 739 faculty and staff members. Overall sanitary conditions were satisfactory, and full-time school physicians were available onsite. All reported cases occurred exclusively among students; no teachers or staff members were affected. The two affected classes were situated on the east side of the same floor within the same building, positioned adjacent to the girls’ restroom where ventilation was observed to be inadequate.
Epidemiological investigation revealed that the index case developed symptoms on September 9, including fever (reaching 38.6 °C), body aches, and nasal congestion. Between September 9 and 11, a total of 15 students from the 2 classes developed fever, with maximum recorded temperatures reaching 39.9 °C. Among these cases, 4 students experienced headache, fatigue, and other systemic symptoms; 3 reported cough and sore throat; 2 had muscle aches; 1 presented with nasal congestion; and 5 exhibited only fever. No hospitalizations, severe cases, or fatalities occurred. The temporal distribution of cases demonstrated a concentrated pattern: 5 cases on September 9, 6 cases on September 10, and 4 cases on September 11. Of the 15 affected individuals, 6 were male and 9 were female, with all students being 6 years old.
A total of 14 throat swab samples were collected from the identified cases. Total viral nucleic acid was extracted using a nucleic acid extraction and purification kit (DA0623, Da’an, Guangzhou) with a nucleic acid extractor (Smart32, Da’an, Guangzhou). Comprehensive screening for respiratory pathogens was performed using a nucleic acid detection kit targeting 22 respiratory pathogens (Beijing Kangrun Gino) in combination with a PCR amplification instrument (TianLong Gentier 96R, Xi’an Tianlong). The screening panel included Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2), influenza A (H1N1) pdm09 and A(H3N2), influenza B viruses, human respiratory syncytial virus, HAdV, human metapneumovirus, human rhinovirus, human parainfluenza virus (HPIV I-IV), human coronavirus (HCoV-NL63, -229E, -OC43, and -HKU1), human bocavirus, human enterovirus, Mycoplasma pneumoniae, Streptococcus pneumoniae, group A streptococcus, Bordetella pertussis, Haemophilus influenzae, Klebsiella pneumoniae, Legionella pneumoniae, Aspergillus, Chlamydia pneumoniae, Chlamydia psittaci, Cryptococcus, and Pneumocystis. HAdV-specific genes were detected in 10 of the 14 samples, while no other pathogens were identified, establishing HAdV as the causative agent of the outbreak.
Six viral isolates were successfully obtained by inoculating the human epidermoid larynx carcinoma cell line (HEp-2 cells) with HAdV-positive samples. Cytopathic effects were monitored daily, and viral material was harvested when greater than 75% of the HEp-2 cells exhibited cytopathic changes. The six viral isolates were subsequently stored at −70 °C for further analysis.
For preliminary genotyping, we amplified and sequenced the Loop2 region of the hexon gene from all six viral strains following national CDC protocols (3) (Table 1). For definitive genotyping, we amplified and sequenced the complete penton base gene (1,635 bp), hexon gene (2,935 bp), and fiber gene (960 bp) (4) (Table 1). All PCR products underwent bidirectional sequencing to ensure accuracy and reliability. We assembled and processed sequence data using Sequencher v5.0 software (Genecode,USA). Phylogenetic trees were constructed using both Neighbor-Joining and Maximum Likelihood (ML) methods in MEGA12, with bootstrap values set to 1,000 replicates for statistical validation. Reference sequences were obtained from the GenBank database.
Primer Primer sequence (5’–3’) The length of the PCR Products (bp) Specificity Reference ad1 TTCCCCATGGCICAYAACAC 482 Loop2 (3) ad2 CCCTGGTAKCCRATRTTGTA HAdV3-Fiber-F CTTCCTACCAGCAGCACCTC 1,210 Fiber (4) HAdV3-Fiber-R CGTGGGGAGAGATTGGTGTA HAdV3-Hexon-1F AGTACTCTGAACAGCATCGT 1,621 Hexon-1 (4) HAdV3-Hexon-1R TAGGTGGCGTGTACTTGTAA HAdV3-Hexon-2F ACCGATGACGCTAATGGATG 1,739 Hexon-2 (4) HAdV3-Hexon-2R TATGGCGCAGGCGAGCTTGT HAdV3-Penton-F AGGACTCTGCCGATGAYAGC 1,846 Penton base (4) HAdV3-Penton-R GATGTAGGCGCAGTAGGAGT Abbreviation: PCR=polymerase chain reaction; HAdV=human adenovirus. Table 1. Primers used to amplify the target genome.
Sequence analysis of the hexon Loop2 region revealed 100% identity among the six viral strains, all clustering with the HAdV-B3 genotype. Further analysis of the complete penton base gene, hexon gene, and fiber gene demonstrated 100% sequence identity among the six isolates. Phylogenetic analysis indicated that the penton base gene was closely related to HAdV-B7 (Figure 1), while the hexon gene and fiber gene were closely related to HAdV-B3 (Figure 1). According to the International Adenovirus Working Group guidelines for HAdV genotype identification (http://hadvwg.gmu.edu), this recombinant profile (P7H3F3) corresponds to the genotype HAdV-B114.
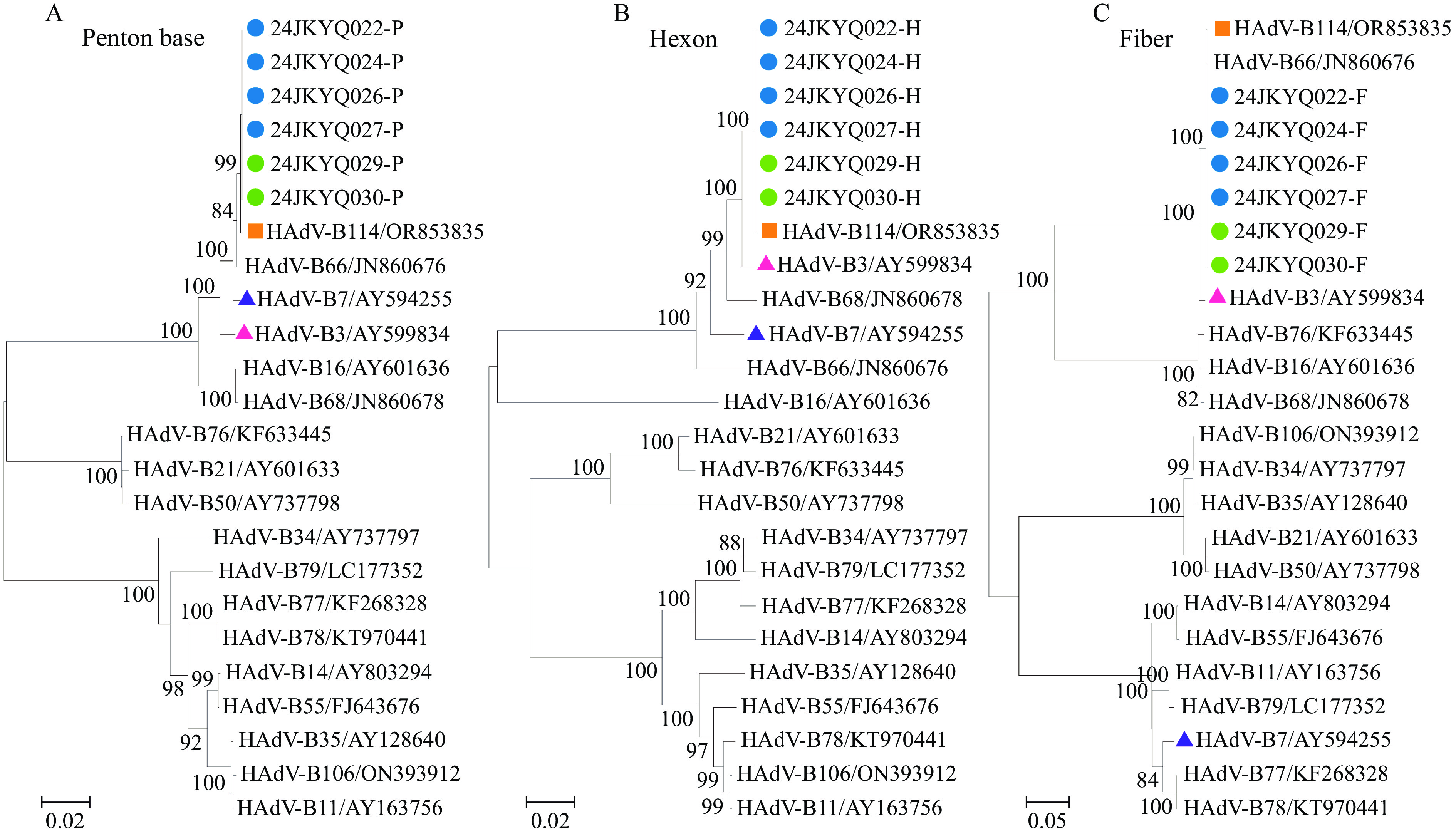 Figure 1.
Figure 1.Phylogenetic tree of HAdV final genotyping based on the penton base gene, hexon gene, and fiber gene.
Note:
Abbreviation: HAdV=human adenovirus.
Further analysis of amino acid sequence differences between the six HAdV-B114 strains revealed that the penton base demonstrated 99.2% amino acid homology with the HAdV-B7 reference strain, the hexon showed 99% amino acid homology with the HAdV-B3 reference strain, and the fiber exhibited 96.8% amino acid homology with the HAdV-B3 reference strain (Table 2). BLAST analysis further confirmed that the penton base gene, hexon gene, and fiber gene of the six HAdV strains exhibited the highest homology with the GenBank reference OR853835 for HAdV-B114 — at 99.9%, 100% and 99.8%, respectively. Therefore, the causative agent of this outbreak was conclusively identified as the novel recombinant HAdV type HAdV-B114.
Strain name Penton base gene Hexon gene Fiber gene 11 32 159 462 141 299 302 411 418 429 439 440 445 22 23 150 207 215 222 223 246 272 316 HAdV-B3/AY599834 − − − − G E N N T T A P T S S Q S E A D H M R HAdV-B7/AY594255 M I V A − − − − − − − − − − − − − − − − − − − 24JKYQ022-P V L A T R G D D R A D T A N L E L Q T H D T T 24JKYQ024-P V L A T R G D D R A D T A N L E L Q T H D T T 24JKYQ026-P V L A T R G D D R A D T A N L E L Q T H D T T 24JKYQ027-P V L A T R G D D R A D T A N L E L Q T H D T T 24JKYQ029-P V L A T R G D D R A D T A N L E L Q T H D T T 24JKYQ030-P V L A T R G D D R A D T A N L E L Q T H D T T Note: “−” means Amino acid site not provided.
Abbreviation: HAdV=human adenovirus.Table 2. Variations in amino acids of human adenovirus type 114 (HAdV-114) strains.
-
According to the Prevention and Control Technical Guide for Respiratory Infections with Human Adenovirus (2019 Edition), in order to better and faster control the epidemic, CDC professionals suggest that schools regularly take temperature screenings, identify absent cases due to respiratory infection, prompt report respiratory infections in students and faculty and staff members, isolate symptomatic infected individuals, and ensure treatment in time. Meanwhile, schools should increase ventilation and disinfection in activity areas, such as classrooms, and provide health education on the prevention of respiratory infectious diseases to students, teachers, and parents. All large gatherings are prohibited under the outbreak of epidemic.
-
Human adenovirus represents a globally prevalent pathogen and constitutes one of the primary etiological agents of acute respiratory infections (ARI) (1–2). Recent years have witnessed multiple reports documenting acute respiratory outbreaks attributed to HAdV (5–7). In September 2024, an acute respiratory outbreak emerged at an elementary school in the Jingkai area. The predominant clinical manifestation among infected children was fever, while other typical respiratory symptoms remained notably absent or mild. The rapid progression from the index case to clustered onset presented diagnostic challenges, making pathogen identification through epidemiological investigation alone insufficient. Laboratory analysis utilizing genetic screening and pathogen identification definitively established that this outbreak was caused by a novel recombinant HAdV-B114 with genotype P7H3F3, representing an evolutionary product of genetic recombination between HAdV-B3 and HAdV-B7.
Domestic and international research demonstrates that HAdV-B3 and HAdV-B7 represent the most prevalent HAdV types causing acute respiratory infections. The six HAdV-B114 strains identified in this study exhibited 100% nucleotide homology across the penton base, hexon, and fiber genes, suggesting that this novel recombinant HAdV-B114 possesses distinct evolutionary advantages and may demonstrate epidemic potential in Beijing. During the 2022–2023 acute respiratory outbreak in Bangladesh, India (7), three HAdV strains with the P7H3F3 genotype were identified, further indicating the widespread prevalence of HAdV-B114. Currently, domestic HAdV surveillance protocols only conduct genotype screening based on the Loop2 region of the hexon gene. Whether HAdV-B114 has achieved widespread circulation in Beijing or throughout China requires retrospective genotype confirmation based on complete penton base, hexon, and fiber gene sequences. This outbreak reinforces the critical importance of comprehensive genotyping based on full-length sequences of these three key genes.
This study’s comparative gene analysis revealed that HAdV-B114 exhibits multiple sequence variations at both the nucleotide and amino acid levels compared to HAdV-B3 and HAdV-B7 across the penton base, hexon, and fiber genes. Given the critical roles these proteins play in viral adsorption, cellular infection, and host immune response modulation, further investigation is warranted to determine how genetic recombination has altered the infectivity and pathogenicity profiles of this novel HAdV-B114 strain. Additionally, the HAdV-B114 isolates obtained in this study demonstrate high nucleotide and amino acid homology with existing reference sequences, indicating that the HAdV-B114 strain identified in Beijing remains within the same evolutionary lineage as the 2023 German isolate.
In recent years, HAdV outbreaks have increased in frequency, accompanied by continuous genetic evolution through recombination (7–8). These evolutionary changes have altered viral tissue tropism and immune evasion profiles, resulting in the emergence of novel strains with enhanced pathogenicity and transmissibility. This phenomenon poses significant challenges to public health systems worldwide. There is an urgent need to establish a comprehensive HAdV surveillance network in China that incorporates genotyping based on complete sequences of the penton base, hexon, and fiber genes. Such enhanced surveillance would strengthen our capacity to identify emerging strains, trace outbreak origins, and improve preparedness for future HAdV-related epidemics.
HTML
| Citation: |



 Download:
Download:




