-
Introduction: On August 13, 2024, the X County CDC reported that multiple villagers in Y Village within a scenic area had developed unexplained generalized pruritic rashes since July 10. Preliminary investigations revealed that most patients had swum in the river prior to symptom onset. The incident generated public concern and negatively impacted local tourism. To identify the cause and control the outbreak, our center conducted an on-site investigation.
Methods: Cases were defined as residents or visitors in Y Village presenting with rash and pruritus without other identifiable causes between July 10 and August 16, 2024. We identified cases through interviews with village doctors, household surveys, and medical record reviews. Thirty village cases comprised the case group, while 60 asymptomatic residents from the same period were randomly selected as controls (1∶2 ratio). Household interviews collected exposure and allergy histories.
Results: We identified 32 cases (30 villagers, attack rate 9.29%; 2 tourists). Village cases ranged from 3 to 77 years of age. The 0–14-year age group exhibited the highest attack rate (26.92%), with a decreasing trend observed as age increased (χ² for trend=16.45, P < 0.001), and rates declining significantly in older groups (χ²=20.86, P<0.001). Symptom onset clustered between July 10 and August 16, peaking on July 20. The median symptom duration was 6 days (range: 3–14 days). All cases presented with rash and pruritus (100%). Village Group A had the highest attack rate at 32.08% (χ²=34.25, P<0.001). Environmental investigation revealed Redoa leucoscela larvae (expert-identified) parasitizing riverside Pterocarya stenoptera trees. Urticating setae from R. leucoscela larvae were detected in river water. Univariate analysis identified significant risk factors as staying under P. stenoptera trees [odds ratio (OR)=14.03, 95% confidence interval (CI): 4.29, 45.87] and swimming in the river (OR=4.60, 95% CI: 1.71, 12.38).
Conclusion: This dermatitis outbreak resulted from dermal contact with R. leucoscela larval urticating setae.
-
On July 10, 2024, Y Village residents in X County, Tongren City, Guizhou Province, developed cutaneous pruritus and erythematous rashes after river swimming. Affected individuals sought treatment at the village clinic, receiving antihistamine therapy. Subsequently, the X County government received successive reports from local and surrounding residents documenting that visitors to the area developed pruritic rashes after their visits, prompting concerns regarding potential environmental triggers. Additional tourists reported similar dermatological symptoms through digital platforms, generating public concern and impacting regional tourism. Given escalating public health implications, local authorities designated this a priority response, mobilizing the X County CDC for comprehensive epidemiological investigations. This investigation retrospectively identified unreported cases among village residents dating to July 10, confirming multiple residents and tourists developed generalized pruritic dermatitis following direct river exposure. On August 13, the X County CDC reported comprehensive findings — including case timelines, tourist complaints, public sentiment impacts, and preliminary results — to the Tongren City CDC. Upon notification, the Tongren City CDC deployed a multidisciplinary investigative team comprising epidemiologists, clinical specialists, and laboratory scientists to conduct a field investigation aimed at verifying the outbreak, elucidating the etiology, and implementing appropriate public health control measures.
-
We defined suspected cases as individuals within Y Village presenting with unexplained pruritus and skin rash between July 10-August 16, 2024, excluding cases with confirmed alternative etiologies. The start date corresponded to symptom onset in the index case, confirmed as the first autochthonous case within Y Village without travel history or documented allergen exposure.
Cases were identified through hospital record reviews, clinician interviews, and active case-finding. All cases met predefined inclusion criteria: symptom consistency, exclusion of alternative dermatoses, and dermatologist-verified diagnoses. We investigated all cases using a Cluster Dermatitis Case Investigation Form collecting clinical manifestations, exposure histories, and demographics. River water quality was assessed per Environmental Quality Standards for Surface Water (GB 3838-2002). We conducted a 1:2 unmatched case-control study to identify risk factors. Associations were analyzed using chi-square or Fisher’s exact tests. We calculated relative risk (RR) with 95% confidence intervals (CI) for age groups (reference: ≥65 years) and odds ratios (OR) with 95% CI for categorical exposures. Statistical significance was P<0.05 using SPSS (version 26.0, IBM Corporation, Armonk, NY, USA).
The outbreak occurred in a scenic area in Y Village, Tongren City, Guizhou Province. The village has seven administrative groups with 323 permanent residents along the river. Boasting a beautiful environment and a temperate climate (mean annual temperature 16 °C), it attracts a large number of tourists .
We identified 30 confirmed cases among Y Village residents, yielding an attack rate of 9.29% (30/323, 95% CI: 6.37, 13.00). Two tourists with similar symptoms were excluded due to insufficient documentation. All 30 cases were investigated using a standardized “Cluster Dermatitis Case Investigation Form”. Contact tracing identified two index cases: Index Case A swam in the river for 30 minutes, then barbecued beneath riverside trees. At 17:00, red rashes with pruritus appeared on limbs and torso. They received a clinical diagnosis of allergic contact dermatitis at X County People’s Hospital and improved with anti-allergy treatment. Index Case B operated a local guesthouse and experienced recurring rashes on torso and limbs with pruritus since mid-July. They reported no river swimming but noted their guesthouse’s proximity to P. stenoptera trees with numerous “caterpillars” congregating — a phenomenon not observed previously. Verified betamethasone use achieved partial symptom resolution.
The primary presentation was pruritic maculopapular rashes predominantly affecting limbs and trunk (Figure 1B). No cases exhibited fever, lymphadenopathy, or systemic involvement.
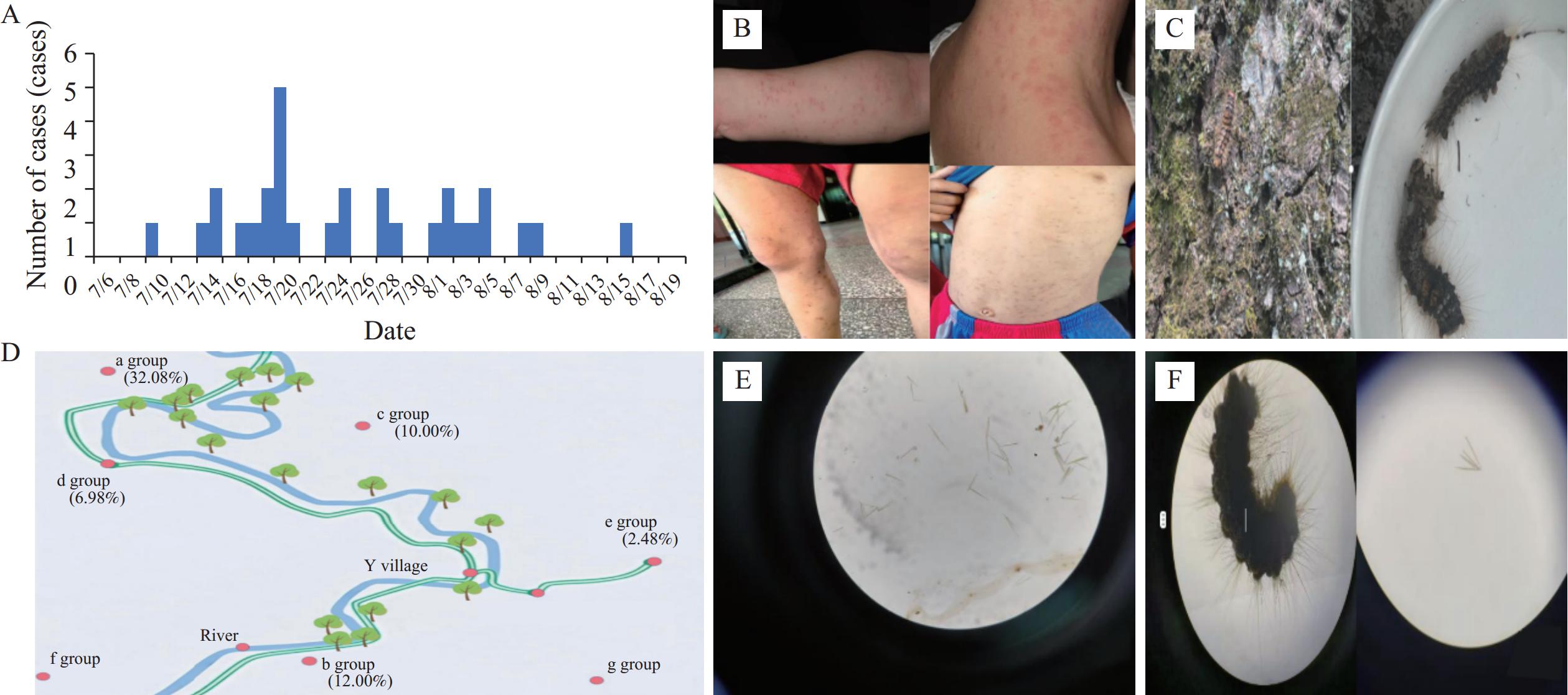 Figure 1.
Figure 1.Overall overview of the 2024 dermatitis outbreak caused by R. leucoscela larvae in a scenic area of Tongren City. (A) Epidemic curve of rash cases in Y Village, July–August 2024; (B) Clinical manifestations of rash cases in Y Village; (C) R. leucoscela larvae observed at the investigation site; (D) Distribution of village groups by disease incidence in Y Village; (E) Morphology of suspected urticating setae from R. leucoscela larvae detected in river water samples; (F) Water sample containing R. leucoscela larvae after one-hour soaking period.
Among 30 confirmed cases, symptom onset occurred between July 10 and August 16, 2024. The epidemic peaked on July 20 (n=5, 16.7%; Figure 1A), with an epidemic curve characteristic of continuous common-source exposure. Cases clustered within five residential groups: a, b, c, d, and e (Figure 1D). Attack rates varied significantly across groups (χ2=34.25, P<0.001), with the highest in a (32.08%) and lowest in e (2.48%).
Case ages ranged from 3 to 77 years. Attack rates showed significant heterogeneity across age groups (χ2=20.86, df=3, P<0.001). The 0–14-year group had the highest attack rate (26.92%), followed by 15–44-year group (20.93%). The 45–64-year and ≥65-year groups showed lower rates (5.88% and 5.08%, respectively). Using RR analysis with ≥65-year group as reference, the 0–14-year group demonstrated highest risk (RR=5.28, P< 0.001), while 15–44-year group showed second-highest risk (RR=4.11, P<0.05). No significant difference emerged between 45–64-year and ≥65-year groups (P>0.05) (Table 1). Sex-based attack rates showed no significant difference: 12.07% in males (21/174) vs. 6.04% in females (9/149) (χ2=3.46, df=1, P>0.05).
Age group (years) Population Cases Attack rate (%) RR (95% CI) vs. ≥65 years P 0–14 26 7 26.92 5.30 (1.94, 14.50) <0.001 15–44 43 9 20.93 4.12 (1.56, 10.88) 0.002 45–64 136 8 5.88 1.56 (0.41, 3.24) 0.78 ≥65 118 6 5.08 1.00 Note: Attack rate calculated as (cases/population)×100%. RR (95% CI) vs. ≥65 years: Relative risk and 95% confidence interval calculated using the ≥65 years group as reference. RR=attack rate of target age group ÷ attack rate of ≥65 years group. Statistical significance defined as P<0.05.
Abbreviation: CI=confidence interval; RR=relative risk.Table 1. Age-specific attack rates of dermatitis in Y Village, 2024.
Based on field observations and preliminary findings, we developed two etiological hypotheses for the clustered dermatitis outbreak in Y Village: 1) waterborne exposure through direct contact with contaminated river water, and 2) dermal contact with urticating setae from “caterpillars” parasitizing P. stenoptera trees.
Environmental surveys revealed no industrial pollution sources near Y Village. The adjacent river contained clear water, with P. stenoptera trees, willows, and other species along both banks. Only P. stenoptera trees exhibited “caterpillar” activity (Figure 1C). Villagers reported unprecedented larval density on riverside trees beginning July 2024 — not observed previously — with larval morphology consistent with documented descriptions. During pre-outbreak tourism development, investigations documented concurrent increases in: 1) visitor density; 2) nighttime illumination duration.
Guizhou Provincial CDC entomology experts identified “caterpillars” inhabiting P. stenoptera trees as R. leucoscela larvae. Two investigators conducted controlled exposure by applying river water to their arms. Within two minutes, both developed red rashes and papules with burning sensations and pruritus — symptoms matching outbreak cases. Symptoms resolved spontaneously upon leaving the contaminated area.
Water quality analysis demonstrated compliance with Surface Water Class II standards (GB 3838-2002). Microscopic examination revealed structures morphologically consistent with R. leucoscela urticating setae (Figure 1E). R. leucoscela larvae immersed in sterile water for one hour shed urticating setae morphologically identical to structures in river samples (Figure 1F).
We conducted a case-control study with all 30 confirmed cases (Y Village residents) and 60 randomly selected unaffected controls (1∶2 ratio). Controls satisfied: 1) absence of target disease confirmed by clinical examination; 2) continuous village residence ≥6 months before outbreak; 3) no immunocompromising conditions or active infections. Demographics were not matched to preserve population heterogeneity.
Univariate analysis evaluated associations between rash development and exposures [river swimming, P. stenoptera trees exposure (>1 minute under trees), home flower cultivation, allergic history]. Significant associations emerged for P. stenoptera trees exposure (OR=14.03, 95% CI: 4.29, 45.87) and river swimming (OR=4.60, 95% CI: 1.71, 12.38) (Table 2), while allergic history (OR=1.00, 95% CI: 0.09, 11.49) and flower cultivation (OR=0.38, 95% CI: 0.04, 3.40) showed no significance. Exclusive P. stenoptera exposure increased rash risk 6.25-fold (OR=6.25, 95% CI: 1.04, 37.67). Combined exposure to P. stenoptera trees and river swimming showed 26.25-fold increased risk (OR=26.25, 95% CI: 5.17, 133.34) (Table 3). River-swimming-only exposure indicated 3.33-fold increased risk, though not statistically significant due to limited sample size (OR=3.33, 95% CI: 0.54, 20.45).
Risk factors Number of exposures (cases) Exposure rate (%) OR (95% CI) Case Control Case Control Exposure to P. stenoptera trees 26 19 86.67 31.67 14.03 (4.29, 45.87) River swimming 23 25 76.67 41.67 4.60 (1.71, 12.38) History of allergies 1 2 3.33 3.33 1.00 (0.09, 11.49) Flower cultivation at home 1 5 3.33 8.33 0.38 (0.04, 3.40) Abbreviation: OR=odds ratio; CI=confidence interval. Table 2. Univariate logistic regression analysis of risk factors for dermatitis in Y Village, 2024.
Exposure to P. stenoptera trees River swimming Cases Controls OR (95% CI) + + 21 10 26.25 (5.17, 133.34) + − 5 10 6.25 (1.04, 37.67) − + 4 15 3.33 (0.54, 20.45) − − 2 25 1.00 Note: “+” indicates presence of exposure (individuals were exposed to P. stenoptera trees or engaged in river swimming); “−” indicates absence of exposure.
Abbreviation: OR=odds ratio; CI=confidence interval.Table 3. Association between environmental exposures and dermatitis in Y Village.
-
Patients presenting with dermatitis received comprehensive anti-allergic and anti-inflammatory treatment protocols. Healthcare providers counseled patients to avoid scratching affected areas to prevent secondary bacterial infections and skin damage. Patients experiencing symptom progression or complications were advised to seek immediate specialized dermatological care at appropriate medical facilities. Targeted insecticide applications were systematically implemented to eliminate R. leucoscela populations throughout the affected area. Concurrent community health education initiatives were delivered to residents, emphasizing the critical importance of avoiding river swimming and prolonged exposure to P. stenoptera trees during peak hazard periods (July–August). These educational efforts promoted enhanced personal protective measures during outdoor recreational activities to minimize direct contact with urticating setae and reduce future exposure risks.
-
This dermatitis outbreak resulted from human exposure to urticating setae of R. leucoscela larvae through direct contact. The epidemiological evidence supports this causal relationship. The outbreak period (July 10 to August 16, 2024) coincided precisely with peak R. leucoscela larval activity (1). The epidemic curve exhibits characteristics consistent with intermittent common-source exposure. During peak larval period (July–August) (1), tourism seasonality increased high-risk behaviors (river swimming, P. stenoptera tree exposure), concentrating human activity in setae-contaminated areas. This temporal convergence of peak exposure and setal density with peak disease incidence strengthens the exposure-disease association. Logistic regression confirmed that exclusive P. stenoptera exposure conferred 6.25 times higher risk (OR=6.25, 95% CI: 1.04, 37.67), while combined exposure increased risk 26.25-fold (OR=26.25, 95% CI: 5.17, 133.34) (Table 3), indicating synergistic effects. In the river-swimming-only subgroup (n=19), association was non-significant (OR=3.33, 95% CI: 0.54, 20.45) due to limited power.
Converging ecological, clinical, and exposure evidence explains this outbreak mechanistically. R. leucoscela larvae exclusively inhabit P. stenoptera trees along Y Village’s riparian zones, feeding on foliage and releasing urticating setae during development (1). These setae contaminate air (wind dispersal) and water (direct shedding), with peak contamination in July–August aligning with larval activity (1-2). Cases presented pruritus, erythema, and papules, consistent with previous studies and the 2009 R. leucoscela-associated outbreak in Daxijiang, Guangxi Zhuang Autonomous Region (2).
All cases presented localized pruritic rashes without fever or exudative lesions, excluding infectious etiologies such as rubella and measles. Laboratory analysis was ruling out general contamination. Critically, microscopic examination established direct causal linkage between setae exposure during swimming and subsequent rash development.
Pronounced age-related risk gradients align with established physiological susceptibility patterns and behavioral exposure differences. The 0–14-year age group demonstrated highest attack rates, consistent with pediatric dermatological vulnerability characterized by thinner stratum corneum, elevated transepidermal water loss, and diminished capacity for neutralizing environmental irritants (3). Prolonged aquatic exposure through recreational swimming substantially increased disease risk among younger participants. The 15–44-year age group exhibited elevated risk through frequent river contact during occupational activities (agricultural work) and recreational pursuits. Reduced risk in the 45–64-year and ≥65-year groups likely reflects decreased participation in high-risk aquatic behaviors.
This outbreak exemplifies a critical nexus between tourism development, ecological disruption, and public health. Anthropogenic factors — particularly tourism infrastructure including persistent nighttime illumination, recreational activities, and riverside barbecuing — disrupt ecosystem equilibrium. The phototactic behavior of R. leucoscela adults toward artificial light sources (4-5) expands geographic distribution and elevates reproductive density. These perturbations (anthropogenic habitat modification) create optimal conditions for R. leucoscela population explosions, culminating in elevated human exposure risks. This reveals eco-tourism dynamics as upstream determinants of disease emergence, extending beyond conventional vector-control frameworks..
This study had several limitations. First, recall bias may have influenced self-reported exposures, potentially inflating odds ratios. Second, case under-ascertainment of mild cases among residents and tourists could have underestimated attack rates while overestimating odds ratios. Third, absence of quantitative exposure metrics (e.g., setae concentration) precluded dose-response analysis, limiting ability to define intervention thresholds. Fourth, incomplete baseline data on meteorological conditions and vegetation dynamics compromised capacity for spatiotemporal risk prediction and targeted intervention planning.
The dermatitis outbreak generated substantial long-term community impacts across multiple domains. First, ecological disruption from R. leucoscela proliferation indicated disrupted interspecies equilibrium, potentially triggering cascading threats to species sharing ecological niches. Prolonged imbalance may reduce biodiversity, simplify ecosystem structure, and compromise environmental resilience. Second, tourism economic recession resulted from diminished attractiveness and declining visitation rates. Peak-season revenue losses strained cash flow, particularly challenging small operators and increasing closure risks. Third, downstream sectoral contraction affected linked industries including hospitality and transport, with reduced hotel occupancy, restaurant patronage, and underutilized infrastructure precipitating broader regional economic destabilization. Fourth, health and demographic consequences included elevated dermatitis risks from chronic exposure to urticating setae, while recurrent outbreaks undermined environmental safety confidence. Prolonged health threats may impair quality of life, exacerbate psychological distress, and accelerate population out-migration.
To achieve a sustainable balance between tourism benefits and ecological conservation, comprehensive multidimensional coordination is essential. We propose integrated approaches: First, strengthen ecological protection through defined conservation boundaries and zoned management strategies. Core ecological areas (including water sources and natural habitats) must be strictly protected by prohibiting high-risk activities such as camping and barbecuing. Scientific boundary delineation between tourist zones and protected areas ensures development activities do not interfere with critical habitats. Second, implement biological monitoring systems utilizing big data analytics to track larval breeding cycles and identify high-risk periods. During these periods, sensitive zones should be temporarily closed to minimize anthropogenic interference while maintaining conservation-tourism balance. Third, enhance regulatory frameworks and strengthen enforcement mechanisms. Establish joint enforcement systems across environmental protection, cultural tourism, and urban management sectors. Target violations such as illegal barbecuing and littering through collaborative governance. Promote “Leave No Trace” principles and advocate behavioral guidelines including eco-equipment use and complete waste removal. Fourth, strengthen public education through comprehensive warning signage at entrances and camping sites to enhance visitors’ understanding of ecological risks and conservation requirements. Fifth, develop comprehensive contingency plans including protocols for rapid case identification, environmental risk assessment, targeted vector control, and public communication for sudden ecological events. These plans should integrate rapid medical-environmental agency coordination to mitigate negative impacts promptly. Through these measures, sustainable equilibrium between economic gains and ecological conservation can be established, securing long-term tourism viability and ecosystem stability.
-
The following institutions for their invaluable support throughout this investigation: Tongren Municipal Center for Disease Control and Prevention, X County Center for Disease Control and Prevention, Tongren People’s Hospital, and Tongren Hospital of Traditional Chinese Medicine. We extend special gratitude to Dr. Zhenzao TIAN (Guizhou Provincial Center for Disease Control and Prevention, Guiyang, China) for providing expert taxonomic identification of R. leucoscela specimens collected during the outbreak investigation.
-
This outbreak investigation was conducted as part of routine public health surveillance activities and does not require formal ethical approval.
HTML
| Citation: |



 Download:
Download:




