-
Non-occupational Post-Exposure Prophylaxis (nPEP) involves a 28-day course of antiretroviral drugs administered to Human Immunodeficiency Virus (HIV)-negative individuals following potential HIV exposure to prevent infection (1-2). PEP was first implemented in 1988 for occupational exposures before expanding to non-occupational contexts, including sexual contact and injection drug use (3). In 2007, the World Health Organization (WHO) recommended antiretroviral drugs for nPEP, initiating global implementation efforts (4).
China adopted nPEP later than many countries, but experienced rapid development in implementation. During 2018–2019, the National Center for Acquired Immunodeficiency Syndrome (AIDS)/Sexually Transmitted Diseases (STD) Control and Prevention (NCAIDS) conducted nPEP pilot studies among men who have sex with men across seven provincial-level administrative regions (PLADs) (5). In 2020, NCAIDS issued the “Technical Guidelines for Post-Exposure Prophylaxis of HIV (Trial)” (6). Subsequently, hospitals across the country established prevention clinics for HIV non-professional exposure. These outpatient services are primarily located in designated hospitals for treating HIV-infected individuals and receiving HIV-negative individuals who have engaged in risky behaviors and experienced exposure within 72 hours, providing them with antiviral drugs to prevent possible infection. On November 1, 2022, an nPEP data information system was launched to collect nationwide outpatient data (7). This study evaluates utilization patterns among HIV nPEP clinic attendees in China, analyzes factors influencing medication adherence and follow-up compliance, and provides evidence to optimize HIV nPEP implementation strategies.
-
As of November 1, 2024, China had established 924 clinics providing nPEP services across all 31 PLADs and the Xinjiang Production and Construction Corp (XPCC). These clinics operate within designated and non-designated antiretroviral treatment (ART) facilities, primary healthcare institutions such as community health centers, and private medical facilities. Each clinic is staffed by personnel from their respective host institutions. Upon presentation, attendees undergo a standardized eligibility assessment, including exposure history evaluation, time-since-exposure documentation, risk stratification, and relevant laboratory testing. Eligible individuals who provide informed consent receive a 28-day, three-drug antiretroviral regimen, typically consisting of (TDF or TAF)+(FTC or 3TC)+(DTG or RAL), with fixed-dose combinations preferred when available. Treatment must be initiated within 72 hours of exposure, and regimen selection is individualized based on the patient’s clinical profile and local drug availability. Priority is given to formulations with fewer adverse effects or combination preparations to enhance medication adherence by reducing pill burden and minimizing side effects. Individuals at risk of HIV exposure can locate nPEP clinic addresses and contact information through the official website of the NCAIDS, WeChat official accounts of disease control agencies, or through referrals from local non-governmental organizations. They must seek consultation and assessment within 72 hours post-exposure. Clinics charge standard medical fees, including costs for HIV testing and hepatic and renal function assessments during medical evaluation. Patients pay for blocking medications according to hospital drug pricing.
-
We analyzed surveillance data from the national HIV nPEP Information System, which captured comprehensive case information nationwide from November 2022 to November 2024. The dataset encompasses sociodemographic characteristics, exposure timing, risk assessment outcomes, exposed population classifications, HIV testing results, patient preferences, 28-day medication regimen completion data, and follow-up information at one and three months post-medication among nPEP clinic attendees.
We defined the standardized 28-day medication regimen — our primary measure for assessing medication adherence — as the initiation of PEP following medical evaluation, with consistent and timely drug intake at the prescribed dosage throughout a continuous 28-day period. The 28-day medication adherence rate was calculated as the number of individuals who completed the standardized 28-day regimen divided by the number of individuals who had taken medication for 31 days or more, multiplied by 100%. Follow-up adherence represents patient compliance with scheduled post-treatment medical visits at healthcare institutions, as directed by their healthcare providers.
-
We conducted all analyses using SPSS 29.0 software [originally developed by SPSS Inc. (Chicago, United States) and is now owned and distributed by IBM Corporation (Armonk, United States)] Skewed measurement data were expressed as medians with interquartile ranges M (Q1, Q3), while categorical data were presented as case numbers and constituent ratios. We assessed group differences using χ2 tests (α=0.05, two-sided) and employed logistic regression models to identify factors influencing medication adherence and follow-up compliance.
-
As of November 1, 2024, a total of 54,108 evaluation records were reported by 924 PEP clinics across 31 PLADs and XPCC in China. The median age of seekers was 31 (26, 37) years, with a median time from exposure to evaluation of 17 (10.2, 34.7) hours; 99.03% sought care within 72 hours. Most help seekers were male (88.63%), classified as high-risk (83.11%), and heterosexual with multiple partners (67.66%) (Table 1).
Factors Total (N=54,108) Initiated medication (N=53,405) Did not initiate medication (N=703) Gender, N (%) Men 47,956 (88.63) 47,340 (98.72) 616 (1.28) Female 6,152 (11.37) 6,065 (98.59) 87 (1.41) Age (years), N (%) <18 438 (0.81) 431 (98.40) 7 (1.60) 18–23 7,310 (13.51) 7,216 (98.71) 94 (1.29) 24–49 43,831 (81.02) 43,266 (98.71) 565 (1.29) ≥50 2,529 (4.67) 2,492 (98.54) 37 (1.46) Time from exposure to evaluation (hours), N (%) <12 15,885 (29.36) 15,603 (98.22) 282 (1.78) 12–23 18,506 (34.20) 18,340 (99.10) 166 (0.90) 24–71 19,192 (35.47) 19,002 (99.01) 190 (0.99) ≥72 525 (0.97) 460 (87.62) 65 (12.38) Exposure risk assessment result, N (%) Low risk 9,139 (16.89) 8,639 (94.53) 500 (5.47) High risk 44,969 (83.11) 44,766 (99.55) 203 (0.45) Exposure population classification, N (%) MSM 10,837 (20.03) 10,562 (97.46) 275 (2.54) Other people* 6,627 (12.25) 6,548 (98.81) 79 (1.19) Heterosexual 36,611 (67.66) 36,264 (99.05) 347 (0.95) Injection drug users 33 (0.06) 31 (93.94) 2 (6.06) Results of Human Immunodeficiency Virus (HIV) testing† , N (%) Negative 54,042 (99.88) 53,405 (98.82) 637 (1.18) Positive 66 (0.12) 0 (0) 66 (100.00) Seeker’s opinion, N (%) Refuse to take medicine 153 (0.28) 17 (11.11) 136 (88.89) Request to take medicine 39,075 (72.22) 38,989 (99.78) 86 (0.22) Follow the doctor’s advice 14,880 (27.50) 14,399 (96.77) 481 (3.23) Medication regimen§ (N=53,405), N (%) TDF+FTC+DTG − 9,515 (17.82) − TDF+FTC+RAL − 1,082 (2.03) − BIC+FTC+TAF − 33,685 (63.07) − Other − 9,123 (17.08) − Note: “−” means this value is missing.
Abbreviation: MSM=men who have sex with men; TDF= tenofovir disoproxil; FTC=emtricitabine; DTG=dolutegravir; RAL=raltegravir; BIC=bictegravir; TAF=tenofovir Alafenamide Fumarate; nPEP=non-occupational post-exposure prophylaxis.
* Other people: those other than MSM, heterosexual with multiple partners, and injection drug users.
† The results of Human Immunodeficiency Virus (HIV) antibody testing obtained during the laboratory examination component of the nPEP clinic eligibility assessment process.
§ Medication regimen: This variable categorizes the three primary antiretroviral therapy regimens currently in use, represented as “TDF+FTC+DTG/TDF+FTC+RAL/BIC+FTC+TAF”.Table 1. Characteristics of nPEP clinic attendees in China: initiators vs. non-initiators, 2022–2024.
Among these individuals, 53,405 (98.70%) initiated PEP treatment. The Biktarvy regimen was selected by 63.07% of patients, with 8,233 (15.42%) low-risk individuals still requesting medication. Among all medication users, 60.34% (30,650) adhered to the standardized 28-day regimen, 57.22% (29,064) completed one-month follow-up, and 44.34% (22,521) completed three-month follow-up. Of the 703 individuals who did not initiate treatment, 66 tested HIV-positive before medication, while 136 refused treatment (101 assessed as high-risk) (Table 1 and Figure 1).
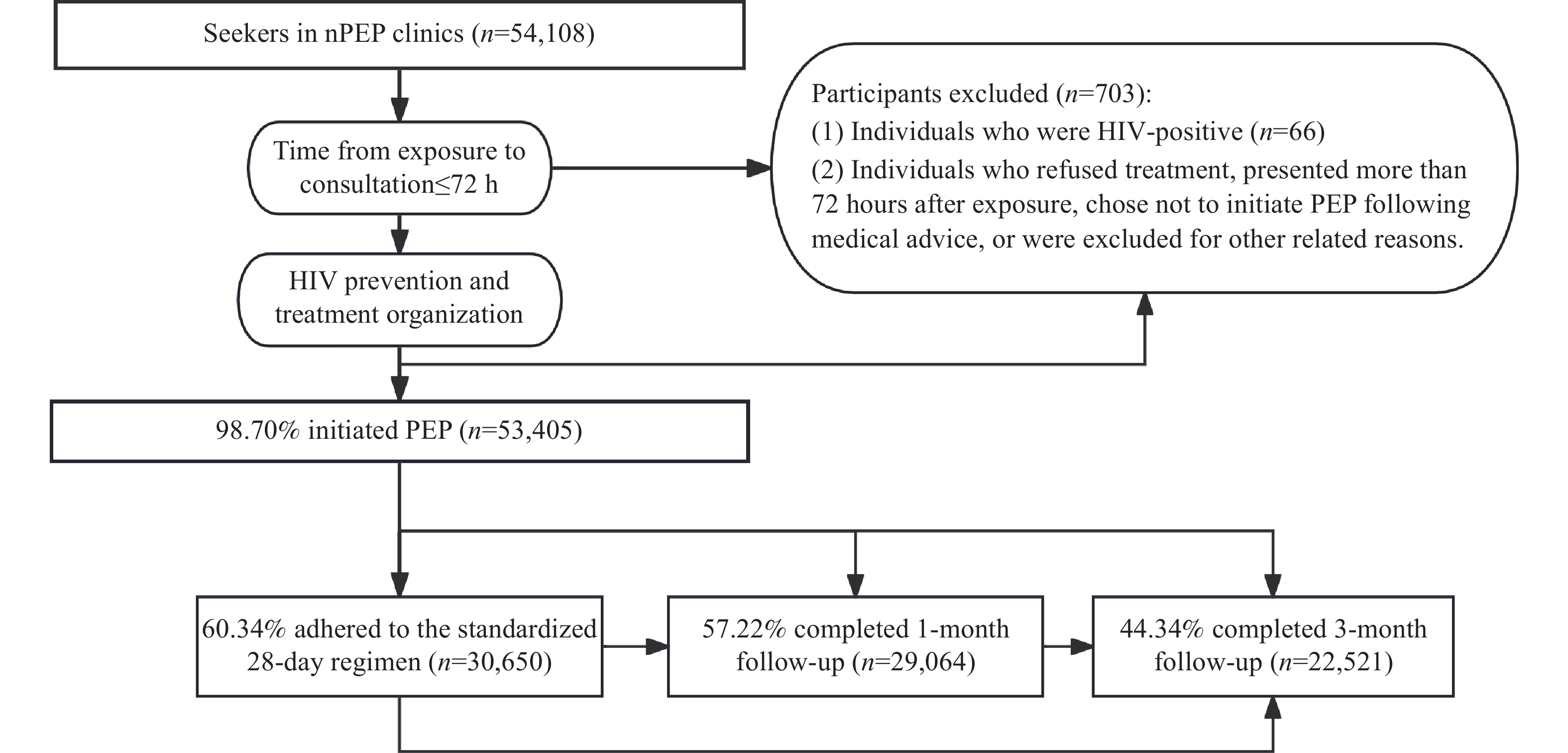 Figure 1.
Figure 1.Flow chart of PEP initiation and follow-up process for nPEP clinic patients in China, 2022–2024.
Abbreviation: PEP=post-exposure prophylaxis; nPEP=non-occupational post-exposure prophylaxis.During follow-up, three users seroconverted to HIV-positive status. Upon verification, one individual engaged in high-risk behavior during treatment, while another sought care more than 72 hours post-exposure, indicating pre-medication infection. Only one case could not rule out PEP failure, yielding a failure rate of 0.0019% (1/53,405).
-
Among medication users, 60.10% (30,509) completed either 1-month or 3-month follow-up visits, while 41.49% (21,076) completed both follow-up assessments. Multivariate logistic regression analysis revealed that high-risk men classified as “other” and those receiving the Biktarvy regimen demonstrated significantly better medication adherence and follow-up compliance compared to other groups (P<0.05) (Table 2).
Characteristics Adhered to the full 28-day
regimen (N=30,650)Adjusted OR*
(95% CI)P Completed 1-month/3-month
follow-ups (N=30,509)Adjusted OR
(95% CI)P Age (years), N (%) <18 247 (0.81) Ref 247 (0.81) Ref 18–23 4,102 (13.38) 1.04 (0.84, 1.27) 0.748 4,084 (13.39) 1.04 (0.85, 1.29) 0.688 24–49 24,812 (80.95) 1.02 (0.83, 1.25) 0.872 24,705 (80.97) 1.03 (0.84, 1.27) 0.757 ≥50 1,489 (4.86) 1.11 (0.90, 1.39) 0.332 1,473 (4.83) 1.11 (0.90, 1.39) 0.332 Gender, N (%) Male 27,274 (88.99) Ref 27,163 (89.03) Ref Female 3,376 (11.01) 0.89 (0.84, 0.95) <0.001 3,346 (10.97) 0.88 (0.83, 0.94) <0.001 Exposure risk assessment result, N (%) Low risk 4,503 (14.69) Ref 4,447 (14.58) Ref High risk 26,147 (85.31) 1.45 (1.38, 1.52) <0.001 26,062 (85.42) 1.48 (1.41, 1.56) <0.001 Exposure population classification, N (%) MSM 6,333 (20.66) Ref 6,326 (20.73) Ref Other people† 3,974 (12.97) 1.18 (1.10, 1.27) <0.001 3,936 (12.90) 1.15 (1.07, 1.23) <0.001 Heterosexual 20,322 (66.30) 0.88 (0.84, 0.92) <0.001 20,227 (66.30) 0.87 (0.83, 0.91) <0.001 Injection drug users 21 (0.07) 1.21 (0.57, 2.59) 0.610 20 (0.07) 1.02 (0.49, 2.14) 0.954 Seeker’s opinion, N (%) Refuse to take medicine 3 (0.01) Ref 5 (0.02) Ref Request to take medicine 22,652 (73.91) 7.83 (2.24, 27.28) 0.001 22,598 (74.07) 4.07 (1.43, 11.58) 0.009 Follow the doctor’s advice 7,995 (26.08) 6.70 (1.92, 23.36) 0.003 7,906 (25.91) 3.32 (1.16, 9.46) 0.025 Medication regimen§, N (%) TDF+FTC+DTG 5,327 (17.38) Ref 5,022 (16.46) Ref TDF+FTC+RAL 539 (1.76) 0.81 (0.72, 0.93) 0.002 416 (1.36) 0.57 (0.50, 0.65) <0.001 BIC+FTC+ TAF 18,955 (61.84) 1.18 (1.12, 1.23) <0.001 18,919 (62.02) 1.35 (1.29, 1.42) <0.001 Other 5,829 (19.02) 1.31 (1.24, 1.39) <0.001 6,152 (20.16) 1.76 (1.66, 1.87) <0.001 Abbreviation: CI=confidence interval; OR=odds ratio; MSM=men who have sex with men; TDF=tenofovir disoproxil; FTC=emtricitabine; DTG=dolutegravir; RAL=raltegravir; BIC=bictegravir; TAF=tenofovir alafenamide fumarate; nPEP=nonoccupational post-exposure prophylaxis.
* Adjusted OR: multivariate logistic regression model OR, adjusting for all other variables in the table.
§ Other people: those other than MSM, heterosexual with multiple partners, and injection drug users.
† Medication regimen: This variable categorizes the three primary antiretroviral therapy regimens currently in use, represented as “TDF+FTC+DTG/TDF+FTC+RAL/BIC+FTC+TAF”.Table 2. Analysis of factors influencing the standard completion of 28-day medication regimen and completion of 1-month or 3-month follow-up among attendees of nPEP clinics — China 2022–2024.
-
This study presents the first comprehensive nationwide evaluation of HIV nPEP clinic utilization patterns and identifies key factors affecting medication adherence and follow-up compliance. Despite achieving a high treatment initiation rate of 98.70%, only 60.34% of patients completed the full 28-day regimen, and merely 60.10% attended either one-month or three-month follow-up appointments. This substantial gap between initiation and completion represents a critical weakness in effective HIV prevention implementation. These deficiencies may compromise prophylactic efficacy and prevent the timely detection of seroconversion, potentially undermining the overall public health impact. Future interventions must prioritize adherence enhancement and follow-up optimization, supported by robust monitoring systems that enable rapid program adjustments.
The nPEP clinics operate primarily within public general hospitals and designated HIV treatment facilities throughout China. With a median consultation time of 17 hours post-exposure and over half of patients seeking care within 24 hours, the system demonstrates good accessibility and timeliness. This performance reflects the extensive geographic coverage provided by 924 nationwide clinics. Many provincial facilities have expanded beyond traditional operating hours, offering both daytime and nighttime services to enable prompt consultation, risk assessment, and prescription following high-risk exposures. However, since optimal nPEP administration should occur within 2 hours post-exposure and must not exceed 24 hours, the 17-hour average delay provides only a 7-hour window within the optimal timeframe. This timing constraint challenges service efficiency and underscores the need for enhanced rapid testing and evaluation capabilities. Expanding geographic coverage and clinic numbers could reduce travel distances for patients, shorten exposure-to-assessment intervals, and enable earlier medication initiation. Facilities lacking 24-hour service capacity should implement shift systems or emergency referral protocols. Medical evaluations should utilize rapid HIV testing kits for immediate assessment while collecting blood samples for confirmatory enzyme-linked immunosorbent assay testing. Liver and kidney function tests should receive urgent laboratory processing. When assessments occur at disease control institutions or social organizations, these facilities should maintain 1–2 days of emergency medication supplies before referring patients to clinics for additional drugs, ensuring medication access within the critical 72-hour window.
A small proportion of high-risk individuals (116/54,108, 0.28%) refused medication, indicating the need for enhanced education about nPEP benefits among high-risk populations. Conversely, a substantial number of low-risk individuals (8,270/54,108, 15.25%) requested medication, likely reflecting risk overestimation and anxiety following potential exposure. Current PEP guidelines suggest nPEP may be inappropriate for low-risk exposures, and indiscriminate use could potentially increase antiviral resistance risk. Future interventions should address patients' psychological concerns while standardizing assessment procedures to enhance precision in PEP utilization and ensure rational medication use.
System data demonstrate that while the national medication initiation rate among nPEP clinic attendees reached 98.70%, medication adherence remains suboptimal — only 60.34% completed the full 28-day regimen. This finding aligns with international studies reporting 65.6% completion [adjusted odds ratio (aOR)=65.6%, 95% confidence interval (CI): 55.6%, 75.6%] (8). However, two Chinese studies reported significantly higher completion rates of 97% and 93.6% (9–10). This discrepancy may stem from their focus on men who have sex with men (MSM) populations, who demonstrate higher nPEP acceptance rates, benefit from effective pre-nPEP education by social organizations, receive “one-on-one” peer support, and access follow-up services through dedicated PEP platforms. These findings suggest that hospital-based nPEP services require enhanced public education, staff training, and comprehensive support services to improve adherence rates. Follow-up compliance also remains inadequate, with only 60.1% completing either 1-month or 3-month follow-ups — lower than rates reported in domestic MSM studies (10). The superior follow-up rates in previous research were largely attributable to peer educator support. Future studies should examine underlying reasons for medication discontinuation and follow-up interruption across diverse populations to develop targeted interventions. However, conducting PEP public education and outreach services through clinical settings may lack precision in content delivery, making it challenging to effectively reach key populations at risk for HIV infection. Innovative community-based approaches may achieve superior results through targeted outreach and adherence interventions. A cohort study examining Pre-Exposure Prophylaxis (PrEP) adherence and persistence among MSM in China found that peer-referred MSM demonstrated higher PrEP adherence and longer treatment persistence (11). This suggests that future community-based pilot programs could explore peer referral systems to link individuals to PEP services, potentially improving PEP adherence rates. Additional studies utilizing crowdsourcing to promote PrEP adherence or leveraging gamified smartphone interventions (O2O-PEP) to facilitate HIV PEP uptake in MSM populations provide valuable models for enhancing PEP utilization (12–13).
Multivariate analysis demonstrates that male participants, high-risk individuals, and those classified as “other people” exhibit significantly higher rates of completing the 28-day medication regimen and demonstrate superior follow-up compliance. Patients prescribed the “BIC+FTC+TAF, Biktarvy” regimen show enhanced adherence rates, likely attributable to its favorable safety profile and reduced pill burden (14). These predictive factors enable early identification of patients at risk for poor adherence, facilitating the development of targeted intervention strategies (15).
Several limitations warrant consideration in interpreting these findings. Information bias may arise from self-reported adherence data, while our analysis excludes high-risk individuals who do not seek consultation services. Additionally, our reliance on outpatient medical records prevents comprehensive analysis of socioeconomic, behavioral, and psychological determinants of adherence. Furthermore, many nPEP seekers obtain medications through online platforms and were not captured in this study, indicating that our results represent only a subset of the broader nPEP user population.
In conclusion, this study reveals that nPEP clinic attendees at public hospitals in China are predominantly young heterosexual males with multiple sexual partners. These individuals typically complete PEP medication assessment within 24 hours following exposure. However, both 28-day medication adherence rates and follow-up compliance remain suboptimal. Critical factors influencing adherence include gender, exposure assessment outcomes, risk classification, patient preferences, and prescribed medication regimen. Identifying these predictive factors enables healthcare providers to characterize individuals with poor adherence patterns and develop targeted interventions to enhance compliance. Further comprehensive investigations are essential to address these challenges and explore evidence-based strategies for improving follow-up rates and medication adherence. Such improvements will optimize nPEP intervention strategies, ensuring that high-risk individuals can effectively prevent HIV infection and reduce HIV transmission within the community.
-
Data from the national nPEP information system were exempt from ethical review requirements.
HTML
Introduction to the Outpatient Work Process of nPEP
Data Source
Statistical Analysis and Data Availability
Characteristics of Attendees of HIV nPEP Clinics
Analysis of Medication Adherence and Follow-up Compliance
| Citation: |



 Download:
Download:




