-
Bacillary dysentery (BD) represents an intestinal infectious disease caused by Shigella infection, characterized by diarrhea, fever, and abdominal pain, with potential blood or mucus in stools (1). The World Health Organization estimates that BD affects over 270 million individuals annually, accounting for 5%–15% of diarrheal diseases worldwide and representing the second leading cause of diarrheal death across all age groups (2). In China, BD is classified as a Class B notifiable communicable disease. Through sustained economic development and proactive public health interventions, marked reductions in both incidence and mortality have been achieved during the past three decades. The incidence rate declined by 93.2% from 35.4 per 100,000 in 2005 to 2.4 per 100,000 in 2024 (3). Despite these achievements, BD outbreaks continue to occur across many regions of China, with frequent reports of cases in collective settings such as schools (4–5). Identifying the sources of infection and determining the contributing factors remains crucial for preventing and controlling these outbreaks. This study analyzes nationwide data on BD outbreaks from 2008 to 2024, aiming to explore occurrence patterns and epidemiological characteristics, define high-risk outbreak scenarios, and generate evidence-based recommendations for enhancing outbreak response strategies.
-
This study extracted BD outbreak data from reports in the China Public Health Emergency Surveillance System, covering January 1, 2008, through December 31, 2024. All reported outbreaks were identified following the Handbook for Prevention and Treatment of Bacillary Dysentery and verified by the responsible county-level CDC, which conducted comprehensive investigations and implemented response measures for confirmed BD outbreaks.
This study extracted epidemiological variables from investigation reports of each outbreak to establish its database. These variables included reporting province, onset dates of index and terminal cases, outbreak setting, transmission route, case counts, exposed population size, reporting date, outbreak closure date, and etiological confirmation results.
This study then described seasonal patterns using a seasonal index, calculated by dividing the average monthly outbreaks by the overall monthly average during the study period. A seasonal index exceeding 1.0 indicates a significant seasonal pattern. It defined outbreak duration as the interval between the first case’s and last case’s onset dates. Response time was defined as the interval between the first case’s onset and the reporting date. This study calculated the attack rate as the number of cases divided by the number of individuals exposed.
Next, this study used frequencies and proportions to describe categorical variables and medians with interquartile ranges (IQR) to characterize numerical variables. The Mann-Kendall trend test assessed trends in case numbers, outbreak size, and duration. This study employed unconditional logistic regression to identify factors associated with outbreak size. Variables with P<0.1 in univariate analysis were included in multivariate analysis, followed by bidirectional stepwise regression (entry P<0.10, removal P>0.05). Statistical analyses were performed using R (version 4.3.3, R Foundation, Vienna, Austria), with two-sided P<0.05 considered statistically significant.
-
Between 2008 and 2024, China reported 176 BD outbreaks comprising 9,854 cases and 4 deaths. The annual number of reported outbreaks demonstrated a fluctuating downward trend with strong negative correlation to year (rs=–0.88, P<0.001). The distribution across time periods showed 53% of BD outbreaks occurred during 2008–2012, 33% during 2013–2017, and 14% during 2018–2022, with no outbreaks reported in 2023 or 2024. The median attack rate was 5.99% (interquartile range: 2.53%, 13.32%) (Figure 1).
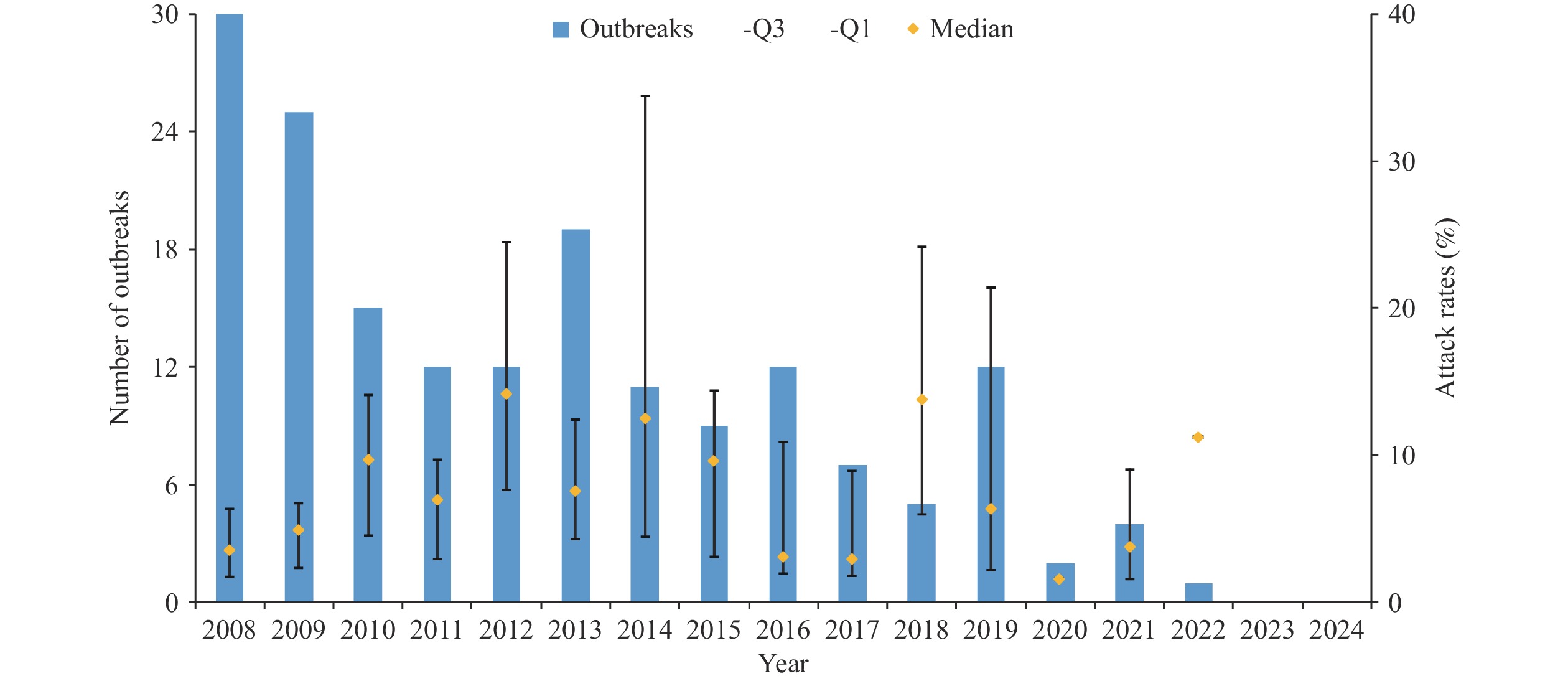 Figure 1.
Figure 1.Annual number of bacillary dysentery outbreaks and corresponding attack rates in China, 2008–2024.
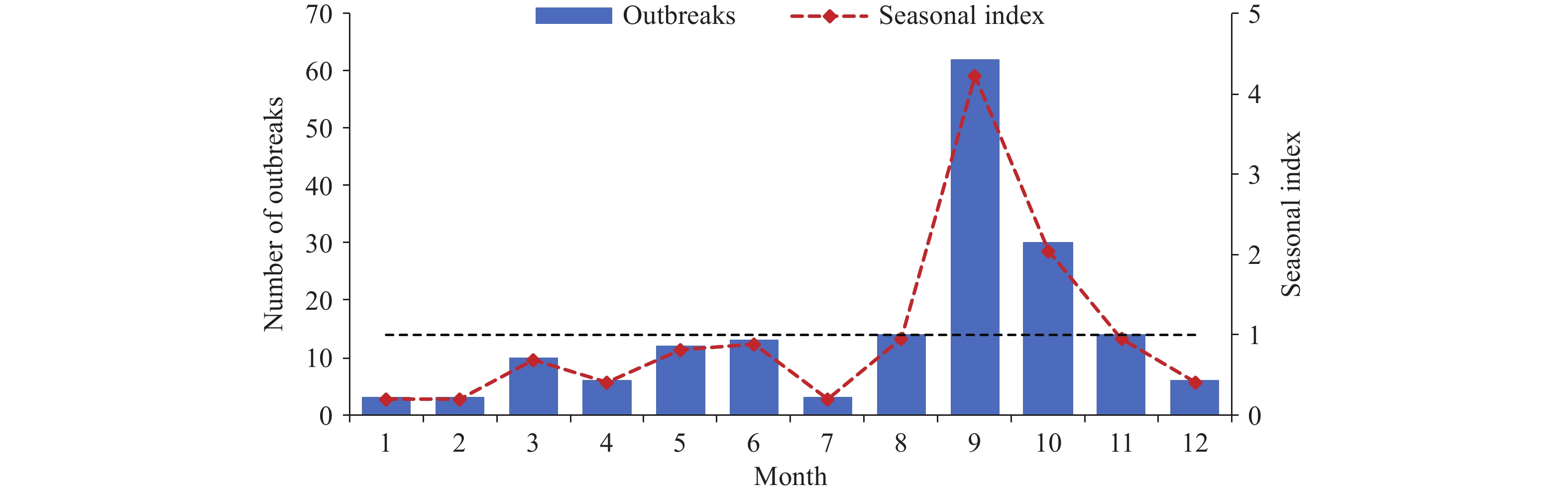
Outbreaks were documented across 102 cities in 22 provincial-level administrative divisions (PLADs). The 5 PLADs with the highest outbreak frequencies were Hunan (46), Guangxi (22), Chongqing (12), Sichuan (12), and Guizhou (11), all situated in southwestern China. While outbreaks occurred throughout the year, 52.3% were concentrated in September and October, with seasonal indices of 4.2 and 2.0 for these months, respectively (Figure 2).
Rural areas accounted for 75.6% of outbreaks (133/176), predominantly occurring in schools (72.2%, 96/133) and villages (24.8%, 33/133). Urban outbreaks represented 24.4% (43/176) of cases, with schools being the most frequent setting (90.7%, 39/43), followed by mental hospitals (4 outbreaks), construction sites (3 outbreaks), and restaurants (1 outbreak). Reporting timeliness differed significantly across settings (H=24.5, P<0.001), with school outbreaks reported within a median of 3 (2,5) days compared to village outbreaks at 7 (4,11) days (Table 1).
Outbreak sites Outbreaks, n (%) Route of transmission, n (%) Pathogen, n (%) Reported time, day
M (Q1–Q3)Waterborne Foodborne Contact-borne Unknown Shigella sonnei Shigella flexneri Shigella boydii Mixed infection Total School 135
(76.7)64
(47.4)34
(25.2)6
(4.4)31
(23.0)79
(79.0)19
(19.0)1
(1.0)1
(1.0)*100
(100.0)3
(2–5)Village 33
(18.8)18
(54.5)6
(18.2)1
(3.0)8
(24.2)4
(25.0)12
(75.0)0
(0.0)0
(0.0)16
(100.0)7
(4–11)Hospital 4
(2.3)1
(25.0)0
(0.0)3
(75.0)0
(0.0)0
(0.00)1
(100.0)0
(0.0)0
(0.0)1
(100.0)16
(8–23)Building site 3
(1.7)1
(33.3)1
(33.3)0
(0.0)1
(33.3)0
(0.0)1
(50.0)0
(0.0)1
(50.0)†2
(100.0)3
(3–6)Restaurant 1
(0.6)0
(0.0)1
(100.0)0
(0.0)0
(0.0)0
(0.0)0
(0.0)0
(0.0)0
(0.0)0
(100.0)2 Total 176
(100.0)84
(47.7)42
(23.9)10
(5.7)40
(22.7)83
(69.7)33
(27.7)1
(0.8)2
(1.7)119
(100.0)3
(2–6)Note: M (Q1–Q3)=Median.
* S. sonnei and S. flexneri.
† S. flexneri and S. boydii.Table 1. Transmission routes, pathogens, and reporting timeliness of bacillary dysentery outbreaks in different settings in China, 2008–2024.
Waterborne transmission predominated, accounting for 47.7% (84/176) of outbreaks. Among waterborne cases, 63 outbreaks (75%) involved drinking water samples exceeding microbial safety standards, while Shigella was successfully isolated from water samples in 34 outbreaks (40.5%). School and village waterborne outbreaks comprised 47.4% and 54.5% of their respective totals. In educational settings, contamination sources included self-supplied well water (73%), secondary water supply systems (8%), water storage tanks (6%), and cafeteria reserve water (6%). Village waterborne outbreaks resulted from contaminated river and spring water exposure through daily activities and vegetable washing during rural gatherings. Foodborne transmission ranked second at 23.9% of outbreaks. The 34 school-based foodborne outbreaks primarily stemmed from contaminated, undercooked, or raw food consumption and poor dietary hygiene. Food handlers or nursery staff were identified as Shigella carriers in 15 outbreaks, while Shigella was detected in stored food samples from 6 outbreaks. Village foodborne outbreaks occurred through consumption of meals at patients' homes and food preparation using contaminated water.
Laboratory confirmation was achieved in 87.5% (154/176) of BD outbreaks. Among the 119 outbreaks with identified pathogen types, S. sonnei (69.7%) was the most frequently detected organism, followed by S. flexneri (27.7%). One S. boydii infection and two mixed infections were also documented.
Using the median number of outbreak cases reported as a cut-off, outbreaks were categorized into large size (cases ≥44) and small size (cases <44) groups for factor analysis. Univariate analysis found that occurrence site, transmission route, response time, duration, and pathogen were associated with outbreak size (P<0.1). Multivariate unconditional logistic regression found that waterborne transmission [adjusted odds ratios (aOR)=5.4, 95% confidence interval (CI): 1.9–15.3], S. sonnei infection (aOR=4.3, 95% CI: 1.7–10.9), and outbreak duration (compared with ≤3 days, aOR for ≥8 days =3.8, 95% CI: 1.0–13.8) were associated with a higher risk of large size outbreaks (Table 2).
Variable Outbreaks, n (%) Univariate Mutivariate OR (95% CI) P aOR (95% CI) P Year 2008–2013 113 (64.2) 1 1 2014–2019 56 (31.8) 0.2 (0.02, 1.8) 0.16 0.2 (0.01, 1.7) 0.12 2020–2024 7 (4.0) 0.2 (0.02, 1.2) 0.08 0.2 (0.01, 1.4) 0.10 Area Urban 43 (24.4) 1 − − Rural 133 (75.6) 1.4 (0.7, 2.8) 0.34 − − Setting Other 8 (4.5) 1 1 Kindergarden 18 (10.2) 4.5 (0.5, 44.4) 0.20 2.1 (0.1, 37.1) 0.61 Primary school 63 (35.8) 14.0 (1.6, 121.4) 0.02 8.3 (0.6, 122.1) 0.13 Middle/High school 42 (23.9) 8.5 (1.0, 75.2) 0.06 4.9 (0.3, 71.0) 0.24 Technical school/College 12 (6.8) 7.0 (0.7, 75.7) 0.11 7.8 (0.4, 150.4) 0.18 Village 33 (18.8) 3.0 (0.3, 28.1) 0.33 2.9 (0.2, 45.4) 0.44 Route of transmission Unknown 40 (22.7) 1 1 Water-borne 84 (47.7) 5.0 (2.2, 11.4) <0.001 5.4 (1.9, 15.3) 0.001 Food-borne 42 (23.9) 2.4 (1.0, 6.0) 0.06 2.6 (0.9, 7.7) 0.09 Contact-borne 10 (5.7) 1.1 (0.3, 5.2) 0.88 1.8 (0.3, 11.4) 0.54 Response time, days ≤3 78 (44.3) 1 1 4–7 56 (31.8) 0.6 (0.3, 1.2) 0.14 0.3 (0.1, 0.8) 0.01 ≤8 42 (23.9) 0.4 (0.2, 0.9) 0.02 0.3 (0.1, 1.1) 0.07 Duration, days ≤3 33 (18.8) 1 1 4–7 62 (35.2) 2.1 (0.9, 5.0) 0.09 2.9 (1.0, 8.5) 0.05 ≤8 81 (46.0) 1.5 (0.7, 3.4) 0.33 3.8 (1.0, 13.8) 0.04 Pathogen Unclassified 57 (32.4) 1 1 Shigella sonnei 83 (47.2) 4.2 (2.0, 8.5) <0.001 4.3 (1.7, 10.9) 0.002 Shigella flexneri 33 (18.8) 1.1 (0.5, 2.8) 0.77 1.5 (0.5, 4.5) 0.44 Shigella boydii/Mixed infection 3 (1.7) 4.0 (0.3, 47.0) 0.27 6.8 (0.3, 138.4) 0.21 Note: - indicates that the variable was not included in the multivariable model.
Abbreviation: aOR=adjusted odds ratios; CI=confidence interval.Table 2. Influencing factors on the outbreak size of bacillary dysentery in China, 2008–2024.
-
This study demonstrates a significant decline in BD outbreaks across China during 2008–2024, consistent with the downward trend in BD cases reported through the national notifiable communicable disease surveillance system (3). This substantial reduction can be attributed to comprehensive nationwide prevention and control measures, including the expansion of centralized water supply systems, enhanced water quality monitoring protocols, implementation of fecal waste treatment and sanitation improvement programs, and increased public hygiene awareness (6). The marked decline observed after 2020 may be associated with non-pharmaceutical interventions implemented during the coronavirus disease 2019 (COVID-19) pandemic, which likely reduced intestinal disease transmission by limiting interpersonal contact and environmental exposure (7).
BD outbreaks were predominantly concentrated in southwestern PLADs. Previous research has identified undulating terrain, favorable climatic conditions, and limited economic development as key risk factors in southwestern China (8). The challenging topography restricts regional economic growth, thereby limiting infrastructure development for safe water supply systems and waste treatment facilities. This results in poor sanitation conditions that facilitate Shigella transmission. Additionally, the temperature and humidity conditions characteristic of southwestern regions create favorable environments for bacterial survival and proliferation. Regional variations in BD reporting requirements may also contribute to observed geographic patterns (9).
Schools emerged as the predominant setting for BD outbreaks. While previous studies have shown that peak BD case reporting typically occurs from June to September (10), this study’s analysis revealed that outbreak reporting peaked in September and October, coinciding with school reopening after summer vacation. The return of students to campus environments increases outbreak risk by elevating bacterial exposure potential through shared facilities and close contact. Therefore, enhanced preventive management measures should be implemented before the academic year begins to mitigate outbreak risk.
Based on epidemiological investigations, this study identified the primary factors associated with outbreak occurrence. The high proportion of waterborne outbreaks aligns with previous findings that link consumable water contamination to disease transmission (9,11). Several schools faced challenges with regular disinfection of self-supplied water sources, while contaminated canteen storage reserves served as pathogen reservoirs, and many students consumed un-boiled water. Schools should implement comprehensive water supply system management and monitoring, ensure student access to boiled or appropriately treated water, and promote awareness of safe drinking water practices. In foodborne outbreaks, Shigella carriage among school canteen staff represented a major cause of institutional outbreaks, emphasizing the critical need for regular health examinations and mandatory valid health certificates for food service personnel. Essential prevention strategies for foodborne outbreaks include enhanced hygiene education in food processing, proper utensil disinfection, strict separation of raw and cooked foods, and thorough cooking procedures.
Laboratory testing revealed that S. sonnei and S. flexneri caused most BD outbreaks, with S. sonnei predominating. This pattern contrasts with results from China’s sentinel surveillance system, where S. flexneri dominates (9,11–12). This study’s findings align with previous reports from China and other economically transitional countries (13), possibly due to the Type VI Secretion System (T6SS) encoded by S. sonnei, which enhances bacterial propagation and survival compared to S. flexneri, thereby increasing the propensity for S. sonnei to cause outbreaks (14).
Pathogen identification rates were substantially lower in other settings (46.3%) compared to school outbreaks (74.1%). Predominant pathogens varied by setting, with S. sonnei more prevalent in schools and S. flexneri in villages. These differences likely reflect environmental factors such as sanitation conditions, population exposure risks, and the biological characteristics of bacterial strains. S. flexneri, characterized by strong virulence, demonstrates better adaptation to environments with poor sanitation typical of rural villages, while S. sonnei, with weaker pathogenicity and milder symptoms, spreads more readily among children and adolescents. Its transmission is further facilitated by the densely populated school environment (15).
Outbreak magnitude varied significantly based on pathogen type and transmission route. Infections caused by S. sonnei, waterborne transmission, and prolonged outbreak duration were more likely to result in large-scale outbreaks. These findings emphasize the critical importance of enhanced drinking water management and the development of healthy consumption habits, particularly avoiding raw water consumption. Furthermore, strengthening surveillance systems and ensuring prompt reporting help reduce epidemic scale, while enhancing laboratory testing capabilities deepens the understanding of epidemiological patterns and antimicrobial resistance profiles, thereby guiding appropriate treatment strategies and reducing BD burden.
This study has several limitations. The data originated from a passive surveillance system, which may have underestimated outbreak frequency due to unreported, undiagnosed, or misdiagnosed cases. Delayed detection can cause reporting delays, while limitations in surveillance sensitivity and geographical coverage may further contribute to missed outbreaks.
In conclusion, this study conducted a comprehensive analysis of BD outbreak characteristics and response measures in China from 2008 to 2024. China has achieved considerable success in controlling bacillary dysentery through effective prevention strategies, with a significant decline in outbreak frequency. China's experience in bacillary dysentery control can provide valuable insights for other developing countries facing similar public health challenges.
-
Sincere gratitude to Lance Rodewald, Senior Advisor at the Chinese Center for Disease Control and Prevention, for his meticulous proofreading and expert language refinement that significantly enhanced the readability and clarity of our manuscript.
HTML
| Citation: |



 Download:
Download: