-
Introduction: On March 20, 2025, a suspected Zika virus (ZIKV) case departed Thailand and flew to Nanning Wuxu International Airport before transiting to Jinan. Upon receiving notification, local CDCs immediately initiated comprehensive epidemiological investigations, laboratory testing, and preventive control measures.
Methods: We collected urine, sputum, and blood samples from the patient for analysis. Quantitative real-time reverse-transcription polymerase chain reaction (qRT-PCR) was employed to detect ZIKV nucleic acid. Metagenome Next-Generation Sequencing (mNGS) was performed on the urine sample to obtain complete viral genome sequences. Phylogenetic analysis was subsequently constructed using the obtained sequences to determine the origin, genotype, and mutation profile of this imported case.
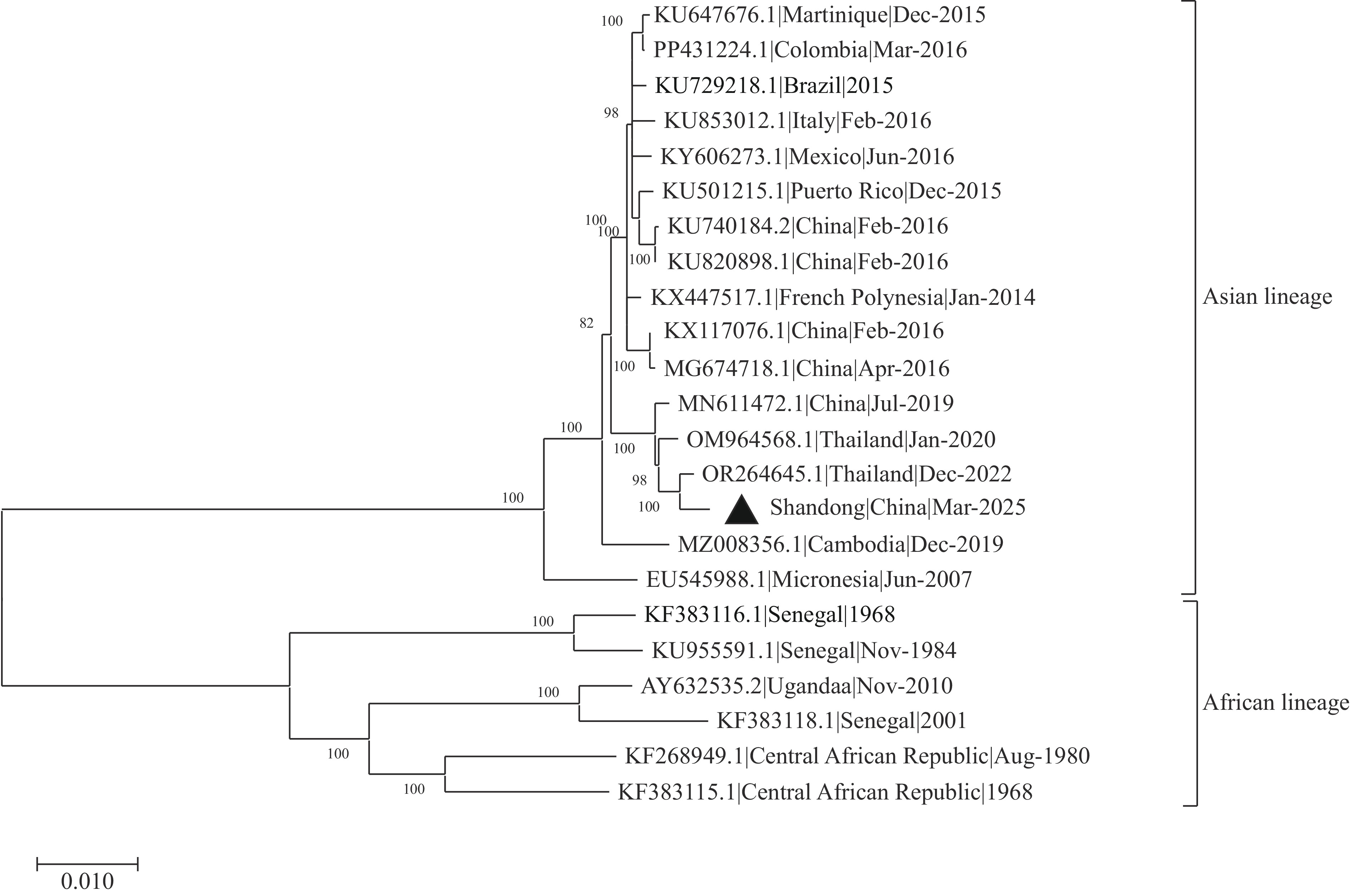
Results: The qRT-PCR analysis confirmed ZIKV presence in the patient’s urine, sputum, and serum samples. The mNGS successfully generated the complete ZIKV genome sequence. Phylogenetic analysis demonstrated that the ZIKV strain belonged to the Asian lineage, exhibiting 99.57% nucleotide homology with a ZIKV strain from Bangkok, Thailand (GenBank accession no. OR264645.1).
Conclusion: Based on the patient’s epidemiological history, clinical presentation, and nucleic acid test results from multiple specimens, this case was confirmed as the first imported ZIKV infection documented in Shandong Province, with the infection source traced to Thailand.
-
On March 21, 2025, the Shandong CDC received an assistance request from Guangxi CDC regarding a suspected ZIKV infection case in a male patient. Upon receiving this notification, Shandong CDC, Jinan CDC, and Tianqiao District CDC immediately initiated case verification procedures, conducted comprehensive epidemiological investigations, and collected sputum, urine, and blood specimens from the patient. Laboratory analysis of the collected samples was performed by Shandong CDC, yielding positive results for ZIKV nucleic acid. Subsequently, on March 23, 2025, China CDC provided official confirmation of the patient’s ZIKV infection status.
-
On February 9, 2025, this 68-year-old male patient traveled alone to Bangkok, Thailand. On March 20, he departed from Thailand and flew to Nanning Wuxu International Airport before connecting to Jinan City, Shandong Province. During thermal screening at the airport, his body temperature measured 37.4℃, and subcutaneous hemorrhagic spots were observed on his chest and arms. He exhibited no arthralgia, myalgia, rash, headache, conjunctival congestion, facial flushing, chest erythema, or neurological symptoms. During his stay in Thailand, the patient reported no sexual activities or blood transfusions, though his history of mosquito bites remained unknown. The patient subsequently returned to his residence in Jinan City on March 21.
On March 20, 2025, airport personnel collected a throat swab on-site at Nanning Airport, which yielded negative results for both the severe acute respiratory syndrome coronavirus 2 antigen test and rapid dengue virus test. Subsequently, throat swabs and blood samples were collected and sent to the Guangxi International Travel Healthcare Center for further validation. On March 21, laboratory results demonstrated that the patient tested positive for ZIKV through quantitative real-time reverse-transcription polymerase chain reaction (qRT-PCR). On March 22, urine, sputum, and blood samples from this patient were collected, and qRT-PCR testing by Shandong CDC confirmed positive ZIKV results in all samples. China CDC Laboratory validated these findings on March 23. The patient was then admitted to the Shandong Provincial Public Health Clinical Center for treatment. On March 23 and 24, qRT-PCR testing of the patient’s urine and sputum samples for ZIKV yielded consistently positive results. However, on March 25 and 26, only the urine samples tested positive for ZIKV, while sputum and blood samples returned negative results. On March 27, qRT-PCR testing indicated that the urine sample remained positive for ZIKV, whereas the sputum sample tested negative. According to the Zika Virus Disease Diagnosis and Treatment Protocol (2nd Edition, 2016), the patient met discharge criteria as blood samples had tested negative for two consecutive days. Consequently, the patient was discharged on March 27. On March 28 and 31, qRT-PCR testing of urine samples returned negative results for ZIKV (Table 1).
Time ZIKV tested by qRT-PCR (Ct) Urine Sputum Blood March 22 (+) 31.24 (+) 32.50 (+) 34.53 March 23 (+) 33.40 (+) 30.05 N March 24 (+) 32.52 − N March 25 (+) 22.57 − − March 26 (+) 31.62 − − March 27 (+) 32.59 − N March 28 − − N March 31 − − N Note: “+” indicates positive result; “−” indicates negative result; “N” indicates no sample collected.
Abbreviation: ZIKV=Zika virus; qRT-PCR=Quantitative real-time reverse-transcription polymerase chain reaction.Table 1. Laboratory test results of biological samples from the patient.
On March 23, 2025, Shandong CDC conducted whole genome sequencing of the patient’s urine sample using Metagenome Next-Generation Sequencing (mNGS) and obtained the complete ZIKV genome sequence. Additionally, we successfully isolated ZIKV from urine samples using Vero (African green monkey kidney) cells, designating the isolate as Shandong|China|Mar-2025. Phylogenetic analysis revealed that this ZIKV strain belongs to the Asian lineage and demonstrates close genetic relatedness to the ZIKV strain from Bangkok, Thailand (GenBank accession no. OR264645.1) (Figure 1), sharing 99.57% nucleotide identity. This ZIKV strain harbors mutations D683E, V763M, and T777M within the open reading frame. Furthermore, an A188V substitution was identified in the NS1 protein.
-
Following confirmation of ZIKV infection, Shandong CDC, Jinan CDC, and Tianqiao District CDC implemented coordinated control measures. Comprehensive disinfection was conducted on the patient’s residence and personal belongings. Vector surveillance was performed in the surrounding environment, revealing no adult mosquitoes or evidence of local transmission. Community residents received instructions on eliminating mosquito breeding sites and implementing personal protective measures.
-
ZIKV has been sporadically documented in northern Africa and Southeast Asia for decades, with only isolated cases reported historically. However, beginning in May 2015, Brazil experienced the largest ZIKV outbreak on record, which rapidly spread throughout multiple South American countries. These widespread outbreaks demonstrated the virus’s potential to cause pregnancy complications, including miscarriage and severe birth defects such as microcephaly. In adults, ZIKV infection can trigger neurological complications, particularly Guillain-Barré syndrome (1). According to the World Health Organization (WHO), 89 countries and territories had reported ZIKV cases as of February 2022 (2). The combination of global population growth, rapid urbanization, and insufficient vector control measures has resulted in an increasing number of countries reporting imported ZIKV cases. These imported cases significantly increase transmission risk in regions where competent vectors, particularly Aedes aegypti and Aedes albopictus, are established.
China confirmed its first imported ZIKV case in Jiangxi Province in February 2016, subsequently followed by additional imported cases in Guangdong Province, Zhejiang Province, Yunnan Province, and other regions (3). On March 21, 2025, Nanning Customs notified Shandong CDC of a suspected ZIKV case involving an individual who had returned from Thailand via Nanning before reaching Jinan. Laboratory confirmation by Shandong CDC and subsequent verification by China CDC established ZIKV infection in this patient, representing the first imported ZIKV case documented in Shandong Province.
Shandong Province, with its dense population in northern China, maintains extensive exchanges in travel, trade, and labor with Southeast Asia. These interactions significantly increase the risk of importing tropical vector-borne diseases. The primary vectors for ZIKV transmission are Aedes aegypti, followed by Aedes albopictus. In Shandong, Aedes albopictus represents the predominant species, typically emerging from late April to early May. When this imported ZIKV case was identified, spring temperatures in Shandong were rising rapidly, with sporadic mosquito activity observed indoors. Consequently, the patient was immediately isolated and treated upon confirmation of infection. Comprehensive disinfection was performed on the patient’s residence and personal belongings. During this process, no adult Aedes mosquitoes were detected, and no local secondary transmission cases were identified.
Most ZIKV cases or asymptomatic carriers typically test negative for ZIKV in serum by the time of detection. This patient’s serum sample demonstrated weakly positive ZIKV nucleic acid results upon entry, indicating the waning phase of viremia. Daily nucleic acid testing of urine, sputum, and blood samples was conducted over one week. The results revealed that ZIKV remained detectable in urine for days 1–6 post-onset, in saliva for days 1–2, and in blood only on day 1. These findings indicated higher viral loads and prolonged shedding in urine compared to saliva and blood, consistent with previous international research (4).
ZIKV is a positive-sense RNA virus belonging to the Flavivirus genus, classified into African and Asian genotypes (5). The molecular evolution of ZIKV correlates closely with its geographical distribution patterns. All documented ZIKV cases in China have been imported from endemic regions, including South America, Oceania, and Southeast Asia. Phylogenetic analysis confirmed that the strain identified in this Shandong case belonged to the Asian lineage, demonstrating the highest degree of homology with reference sequence OR264645.1, which originated from Bangkok, Thailand. This molecular evidence provides definitive support for the conclusion that the patient acquired ZIKV infection during travel in Thailand. Whole-genome sequencing revealed mutations (D683E, V763M, T777M) that have been associated with potential teratogenic effects, including congenital Zika syndrome (6). Additionally, an NS1 protein mutation (A188V) was detected, which may enhance viral transmission capacity and infectivity (6). However, the S139N mutation, previously linked to prolonged neuroinflammation and immune modulation, was absent in this strain (7).
Beyond mosquito-borne transmission, recent years have witnessed an increasing number of documented sexual transmission cases (8), further emphasizing the critical importance of comprehensive ZIKV prevention and control strategies. This case underscores the imperative to strengthen port quarantine measures, particularly during peak travel seasons to Southeast Asian destinations. Essential strategies must be implemented to prevent imported cases through enhanced temperature screening protocols and expanded laboratory testing capabilities for returning travelers at key entry points. Furthermore, during periods of active mosquito activity in southern China, vector surveillance and control efforts require particular intensification. Public education campaigns targeting travelers returning from epidemic regions should be expanded to promote improved personal protective measures. Pregnant women should be specifically advised to avoid travel to ZIKV-affected areas to minimize the risk of congenital anomalies. The successful management of this outbreak demonstrates the effectiveness of close collaboration among health departments, cross-regional disease control agencies, and customs authorities, enabling timely detection of Shandong’s first imported ZIKV case and successfully preventing epidemic spread. This experience reinforces the necessity of establishing normalized multi-sectoral coordination mechanisms that facilitate data sharing and develop real-time infectious disease early warning systems, thereby creating a comprehensive infectious disease prevention and control network that provides robust protection for public health and safety.
-
Approval by the Ethics Committee of Shandong Center for Disease Control and Prevention, China (approval number: 2021-24).
HTML
Investigation and Results
Public Health Response
| Citation: |



 Download:
Download:




