-
Tick-borne diseases (hereafter referred to as TBDs), which are transmitted through ticks as vectors, are characterized by their high infectivity and wide transmission range. Recent years have witnessed a notable increase in the global incidence of these diseases (1-2), along with rising cases of coinfections with multiple tick-borne pathogens (3-4). These diseases pose serious health risks and economic burdens to both humans and animals worldwide, underscoring the critical importance of research in this field. Ongoing processes of globalization, climate change, and increased human-animal interactions have further accelerated the global spread of TBDs, leading to the emergence of new diseases (5-6). China’s complex terrain, combined with diverse tick species and pathogens, presents a major public health challenge. Urbanization and afforestation projects complicate the distribution of ticks and pathogens, creating significant obstacles for disease prevention and control efforts in the country (7). Despite the diversity and complex distribution of tick species in China, the health threats posed by TBDs are often underestimated, hampering current prevention and control initiatives. By analyzing research literature, we can develop a better understanding of the characteristics of various TBDs in China to improve preventive measures.
-
A systematic literature review was conducted using Embase, PubMed, Scopus, and the Web of Science Core Collection. The search terms included ‘Tick-borne diseases’ OR ‘Lyme borreliosis’ OR ‘Tick-borne encephalitis’ OR ‘Q fever’ OR ‘Babesiosis’ OR ‘Crimean-Congo haemorrhagic fever’ OR ‘African swine fever’ OR ‘Tularemia’ OR ‘Kyasanur forest disease’ OR ‘Colorado tick fever’ OR ‘Ehrlichiosis’ OR ‘Anaplasmosis’ OR ‘Rocky Mountain spotted fever’ OR ‘Southern tick-associated rash illness’ OR ‘Tick-borne relapsing fever’ OR ‘Pacific coast tick fever’ OR ‘Omsk haemorrhagic fever’ OR ‘Rickettsiosis’ OR ‘Spotted fever group rickettsiosis’ OR ‘Novel bunyavirus infection’ OR ‘Piroplasmosis’ OR ‘Oriental spotted fever’ OR ‘Relapsing fever borrelioses’ OR ‘Powassan disease’ OR ‘Powassan encephalitis’ OR ‘Imported tick-borne spotted fever’ OR ‘North-Asia spotted fever’ OR ‘African tick bite fever’. As China CDC Weekly is an international peer-reviewed journal that ensures research reproducibility, this study included only English-language publications. The review was limited to research articles published between 2003 and 2023, excluding reviews, case reports, commentaries, interviews, letters, and editorials. Mite-, flea-, and louse-borne diseases, such as Tsutsugamushi, murine rickettsiosis, and infections caused by Rickettsia felis, Rickettsia akari, and Rickettsia prowazekii, were excluded. After removing duplicate articles using EndNote and Excel, only studies related to TBDs were included.
-
We systematically processed data on TBDs from the articles that met the inclusion criteria. These data include geographical origins of cases, incidence and distribution, clinical characteristics, diagnostic methods, and tick vectors.
-
A total of 3,483 articles were initially retrieved from Embase, PubMed, the Web of Science Core Collection, and Scopus. After screening, 67 relevant articles specifically related to TBDs were retained for inclusion in the study. The detailed screening process is illustrated in Figure 1.
 Figure 1.
Figure 1.Flowchart illustrating the search and screening process for articles on tick-borne diseases in China.
Analysis of these 67 articles revealed temporal trends in TBD cases (Figure 2A). The number of patients with severe fever with thrombocytopenia syndrome (SFTS) gradually increased in 2020, peaked in 2021, and then progressively declined in subsequent years. A summary of articles and cases related to various TBDs (Figure 2B) showed that 43% of the articles focused on SFTS, 18% on spotted fever group rickettsiosis (SFGR), and 14% on human granulocytic anaplasmosis (HGA). This finding indicates a shift in research attention toward TBDs in China, with SFTS attracting significant interest among researchers. Furthermore, SFTS accounted for the greatest number of cases, representing 66% (10,080/15,261) of the total TBD cases reported in the country. This underscores SFTS as the most prominent health concern among TBDs in China.
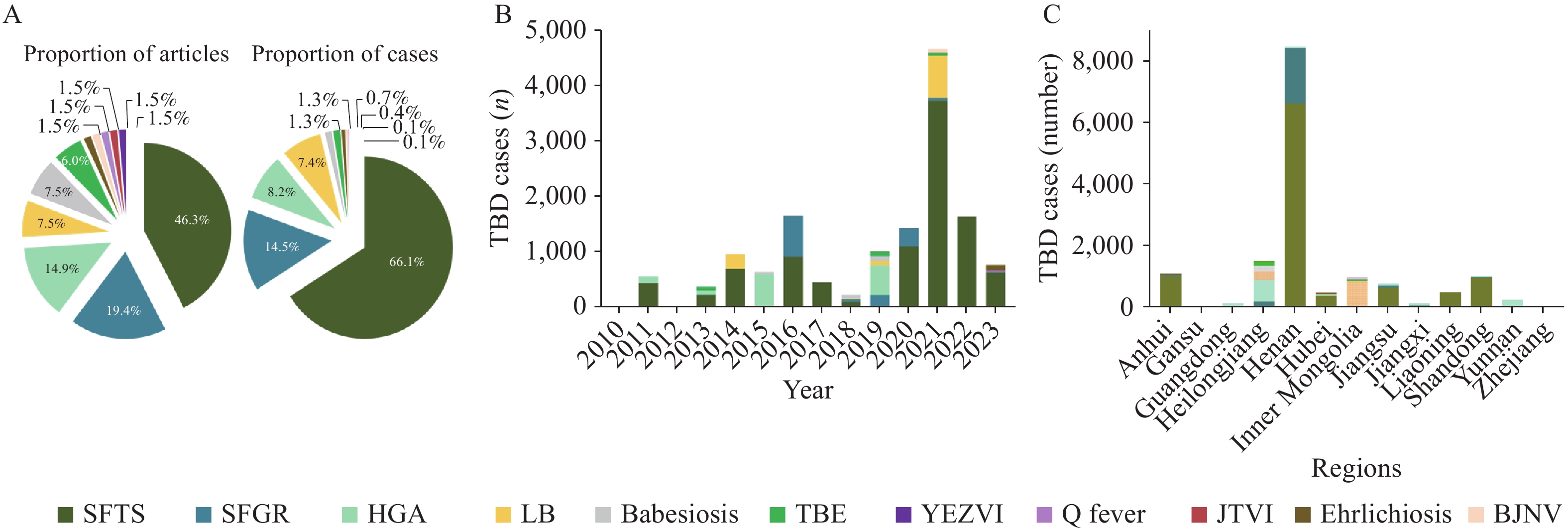 Figure 2.
Figure 2.Distribution of articles and yearly cases of tick-borne diseases in China, 2003–2023. (A) The proportion of articles and cases related to tick-borne diseases; (B) Number of cases per tick-borne disease across the years; (C) Incidence of tick-borne diseases reported across Chinese mainland.
Abbreviation: SFTS=severe fever with thrombocytopenia syndrome; SFGR=spotted fever group rickettsiosis; HGA=human granulocytic anaplasmosis; LB=Lyme borreliosis; TBE=tick-borne encephalitis; YEZVI=Yezo virus infection; JTVI=Jingmen tick virus infection; BJNV=Beiji nairovirus. -
We assessed the geographical distribution of TBD cases across Chinese mainland (Figure 2C). This analysis revealed a concentration of TBD cases primarily in provincial-level administrative divisions (PLADs) such as Henan, Heilongjiang, Anhui, Shandong, Inner Mongolia, and Jiangsu. These findings align with the known distribution of ticks and tick-borne pathogens (7). Additionally, SFTS alone accounted for 10,080 of the 15,261 total TBD cases reported in China, followed by SFGR and HGA, which accounted for 2,212 and 1,257 cases, respectively. Notably, 61.2% (6,611/10,800) of SFTS cases and 81.8% (1,810/2,212) of SFGR cases were reported in Henan Province, 37.9% (477/1,257) of HGA cases were reported in Heilongjiang Province, and 68.5% (771/1,126) of Lyme borreliosis (LB) cases and 74.9% (149/199) of tick-borne encephalitis (TBE) cases were reported in Inner Mongolia Autonomous Region.
A comprehensive categorization of TBDs into viral, bacterial, and parasitic types is presented in Table 1. The clinical characteristics, diagnostic methods, and tick species for these diseases are summarized. In China, Haemaphysalis longicornis, Ixodes persulcatus, and Haemaphysalis yeni serve as the primary vectors for numerous tick-borne pathogens. This prevalence might be attributed to the favorable environmental conditions in China that are conducive to the proliferation of these particular tick species (7).
Category TBD Pathogen Clinical symptom Biochemical indicator Identification method Tick species Citation Viral diseases SFTS Severe fever with thrombocytopenia syndrome virus Fever, anorexia, headache, muscular soreness,
diarrhea, dizziness, ecchymosis, arthrodynia,
central nervous system symptomsThrombocytopenia,leukocytopenia,
elevated aminotransferase levels, elevated lactate dehydrogenase levels, proteinuria, hematuresisRT-PCR H. longicornis (8–41) TBE Tick-borne encephalitis virus Fever, headache,
central nervous system symptomsIncreased white blood cell count, neutrophilic
leukocytosis, elevated aminotransferase levelsPCR NA Jingmen tick virus infection Jingmen tick virus Fever, headache, myodynia, pruritus,
eschar, lymphadenectasisElevated aminotransferase levels, neutropenia RT-PCR I. persulcatus Yezo virus infection Yezo virus Fever, headache, dizziness, diminution of vision,
chest distress, breathe hard, arthrodyniaLymphocytopenia, neutrophilic leukocytosis,
elevated aminotransferase levelsRT-PCR I. persulcatus Beiji nairovirus infection Beiji nairovirus Fever, headache, redness and swollen,
pruritus, depression, coma, fatigue, myalgia,
arthralgia, poor appetite, skin rashes, petechiaeThrombocytosis, leukocytosis, elevated level of high
sensitivity C-reactive protein, elevated aminotransferase
levels, elevated lactate dehydrogenase levelsPCR NA Bacterial diseases HGA Anaplasma phagocytophilum Fever, headache, dizziness, weakness, malaise, anorexia, nausea, chills, cough, abdominal pain, myalgia, ecchymosis, rash, lymphadenectasis Leukocytopenia, thrombocytopenia, elevated aminotransferase levels, elevated lactate dehydrogenase levels PCR, IFA H. longicornis (42–60) SFGR Rickettsia rickettsii Fever, headache, asitia, weakness, cough,
dizziness, rash, eschar, lymphadenectasisThrombocytopenia, leukocytopenia, elevated aminotransferase levels PCR, IFA H. yeni,H. longicornis LB Borrelia burgdorferi Erythema migrans, fever, headache, weak, naupathia, arthrodynia, asitia, lymphadenectasis, erythra, eschar Elevated aminotransferase levels, thrombocytopenia,
leukocytosisPCR NA Ehrlichiosis Ehrlichia spp. Fever, rash, asthenia, anorexia, myalgia Thrombocytopenia, leukocytopenia, elevated aminotransferase levels, increased C-reactive protein PCR H. longicornis Q fever Q fever Rickettsia Fever, headache, muscular soreness, erythra, eschar Thrombocytopenia, elevated high-sensitivity C-reactive protein levels, elevated aminotransferase levels PCR NA Parasitic diseases Babesiosis Babesia spp. Fever, headache, dizziness, naupathia, chills, arthralgia, lymphadenectasis, flu-like symptoms Thrombocytopenia, leukocytosis, elevated amino-
transferase levels, elevated bilirubin levelsPCR NA (61–64) Abbreviation: TBD=tick-borne disease; SFTS=severe fever with thrombocytopenia syndrome; TBE=tick-borne encephalitis; BJNV=Beiji nairovirus; HGA=human granulocytic anaplasmosis; SFGR=spotted fever group rickettsiosis; LB=Lyme borreliosis; RT-PCR=reverse transcription polymerase chain reaction; PCR=polymerase chain reaction; IFA=immunofluorescence assay; H.=Haemaphysalis; I.=Ixodes; NA=no relevant results found. Table 1. Characteristics of TBDs reported in China, 2003–2023.
-
SFTS is a recently identified infectious disease that originated in China and is caused by a novel bunyavirus (65). In China, SFTSV has been detected in Haemaphysalis longicornis ticks collected from livestock in areas where SFTS patients reside, establishing this species as the primary transmission vector (66). SFTS cases in China are primarily concentrated in Liaoning Province in northeastern China; Shandong Province along the eastern coast; Jiangsu and Zhejiang provinces in eastern China; and Henan, Hubei, and Anhui provinces in central China (7). According to this study, a total of 10,080 SFTS cases have been identified in China. The characteristic clinical manifestations include acute fever, fatigue, thrombocytopenia, leukopenia, and gastrointestinal symptoms such as nausea, vomiting, and diarrhea. In severe cases, multiple organ dysfunction may occur (10). Currently, no specific treatment or vaccine is available for this disease, making effective prevention measures crucial in endemic areas (66).
-
SFGR is a zoonosis caused by bacteria of the genus Rickettsia and is transmitted primarily by arthropod vectors, particularly ticks (67). This study identified 2,212 SFGR cases reported in China. The clinical presentation typically includes fever, rash, and other characteristic features such as eschars and lymphadenopathy (68). Early diagnosis and treatment are essential, with antibiotics commonly used to alleviate symptoms and prevent disease progression. Preventive measures include avoiding tick bites, practicing good personal hygiene, and maintaining clean living environments. Our analysis revealed that Haemaphysalis yeni and H. longicornis serve as the primary vectors for SFGR in China.
-
HGA is an infectious bacterial disease caused by Anaplasma phagocytophilum (69). The primary transmission route is through tick bites, with blood transfusion representing another documented mode of transmission (70–71). This study identified 1,257 HGA patients in China. Typical clinical manifestations include fever, headache, myalgia, fatigue, nausea, vomiting, general malaise, and thrombocytopenia with associated bleeding abnormalities (42). HGA cases in China are primarily concentrated in the central and southeastern regions (72). The Infectious Diseases Society of America (IDSA) recommends empiric treatment with doxycycline for all patients with suspected HGA (73). In China, Haemaphysalis longicornis serves as the primary vector for HGA.
-
LB is a naturally occurring epidemic disease caused by Borrelia burgdorferi and is transmitted primarily by ticks, particularly those in the genus Ixodes (74). Although LB is the most commonly reported tick-borne illness in the United States, China has reported fewer cases, with a total of 1,126 documented in this study (7,75). The principal clinical manifestations include skin rash, erythema migrans, fever, fatigue, headache, and arthralgia. Most patients recover with effective treatment, and fatal outcomes are rare (76).
-
Babesiosis is a zoonotic parasitic disease caused by Babesia, a blood protozoan that infects red blood cells and is transmitted to humans and animals through tick bites (77,78). Over the past 20 years, more than 100 patients in Zhejiang, Yunnan, Guangxi, and other Chinese PLADs have been infected with Babesia microti, suggesting that B. microti from field mice may be the primary pathogen causing human babesiosis in China (79). Clinical manifestations include fever, headache, myalgia and arthralgia, cutaneous pain or pruritus, lymphadenopathy, abdominal pain, diarrhea or constipation, dyspnea, anemia, and neurological symptoms such as dizziness, nausea, and blurred vision (80). While typically mild to moderate in severity, babesiosis can lead to severe outcomes or even death in elderly or immunocompromised patients (81).
-
TBE, also known as forest encephalitis, is an acute infectious disease of the central nervous system caused by the tick-borne encephalitis virus of the genus Flavivirus. It is transmitted primarily through the bite of ticks in the genus Ixodes (82). After entering the body via a tick bite, the virus replicates and migrates to draining lymph nodes before spreading to the bloodstream and crossing the blood-brain barrier, resulting in central nervous system damage (83). Between 2007 and 2018, 3,364 cases were reported in Chinese mainland, predominantly in forested areas of northeastern China (84). The principal symptoms include fever, headache, myalgia, fatigue, nausea, vomiting, diarrhea, rash, and, in severe cases, neurological manifestations such as altered consciousness, seizures, and paralysis (85).
-
Due to China’s vast geographical expanse and complex natural environment, combined with the diversity of tick vector species, pathogen types, and intermediate hosts, there likely exist even more types of tick-borne diseases (TBDs) than currently documented. This presents significant challenges for TBD prevention and control efforts in China. Research priorities regarding TBDs in China have evolved over time. Historically, diseases such as Crimean-Congo hemorrhagic fever, African swine fever, Q fever, and Lyme disease were the primary focus (86). However, SFTS has received considerable attention in recent years. SFTS is an emerging tick-borne infectious disease first reported in China in 2009 and subsequently identified as a novel bunyavirus in 2010 (87). SFTS is particularly lethal, with the elderly patients and those with underlying conditions being susceptible to multiple organ failure following infection (36,88). Currently, there is no specific antiviral treatment or approved vaccine for SFTSV (89). The number of SFTS cases increased gradually in 2020, peaked in 2021, and subsequently decreased each year thereafter. This trend may be attributed to the focus on COVID-19 prevention and control efforts, which diverted attention from SFTS surveillance and management. Additionally, the significant fluctuation in TBD incidence in recent years indicates that monitoring and control measures should be strengthened. The clinical features of TBDs underscore the urgency of addressing these diseases. Furthermore, pathogens of many TBDs, including bacteria and viruses, are transmitted primarily by H. longicornis, a tick species widely distributed throughout China (90). Recent research has also shown that Rhipicephalus sanguineus may serve as a vector for SFTSV (91). TBD cases are concentrated in developed agricultural or forestry areas such as Henan, Heilongjiang, and Inner Mongolia. The prevalence of TBDs in these regions is primarily due to favorable environmental factors, including abundant vegetation and livestock resources. These conditions not only provide suitable habitats for the complete tick life cycle from eggs to adults but also promote extensive agropastoral activities within local communities, further exacerbating disease spread (7,92). These findings suggest that urgent measures are needed to control ticks and tick-borne pathogens.
A limitation of this study is that the literature search was restricted to English-language articles, potentially overlooking studies published in other languages.
-
In summary, due to the rich diversity and widespread distribution of tick species in China, TBDs have become a significant threat to public health. Research on these infections, particularly SFTS, continues to face notable challenges. We urge the use of an integrated One Health approach, at the human-animal-environment interface, to enhance prevention and control measures for TBDs, particularly in regions with developed agriculture and forestry, as well as agropastoral activities.
HTML
Search Strategy
Data Processing
Literature Screening
Distribution of TBDs in China
Major Tick-borne Diseases in China
SFTS
SFGR
HGA
LB
Babesiosis
TBE
| Citation: |



 Download:
Download:




