-
Introduction: Current strategies for chronic viral hepatitis prevention and control include immunization, prevention of mother-to-child transmission, expanded testing, antiviral therapy, and national drug price negotiations. To achieve this effectively, Shanghai has implemented a community-based pilot program that integrates public health and clinical care for chronic viral hepatitis management.
Methods: This study evaluated the effectiveness of Shanghai’s community-based healthcare program at three time points (2012, 2019, and 2023), assessing key indicators including antiviral treatment rates and disease status changes and risk of hepatocellular carcinoma. Data were managed using EpiData 3.1, with descriptive statistics and chi-square tests performed using SPSS 29.0.
Results: The study enrolled 1,478, 1,901, and 7,714 patients in 2012, 2019, and 2023, respectively. During the management period, the number of enrolled patients increased substantially from baseline. The antiviral treatment rates in 2019 and 2023 reached 64.5% and 58.2%, with both significantly higher than the baseline rate of 24.5% in 2012. Concurrently, abnormality rates for hepatitis B virus deoxyribonucleic acid (HBV DNA), alanine aminotransferase (ALT), total bilirubin (TBIL), and fibrosis indices decreased significantly in 2019 and 2023. The 2023 aMAP score further revealed a decline in hepatocellular carcinoma risk among managed patients (32.2% vs. 26.3%). With enhanced community healthcare capacity, 14.1% (2019) and 18.2% (2023) of patients accessed community dispensing services, aligning with the strategy to decentralize testing and treatment for disease elimination.
Conclusions: Community-based healthcare management for chronic hepatitis in Shanghai provides patients with decentralized hepatitis-related testing and treatment services, creating an effective environment for chronic viral hepatitis prevention and control and would favorable for the viral hepatitis elimination efforts.
-
To reduce the burden of disease caused by viral hepatitis, the World Health Organization (WHO) adopted the Global Health Sector Strategy on Viral Hepatitis 2016–2021 (GHSS) in 2016, which explicitly set out to achieve “a 90% reduction in incidence (95% for Hepatitis B virus and 80% for Hepatitis C virus) and 65% reduction in mortality by 2030, compared with a 2015 baseline” (1). In response to this goal, integrating the Hepatitis B vaccine (HepB) for children into the Expanded Programme on Immunization (EPI) (2) and the prevention of mother-to-child transmission (MTCT) have reduced the HBsAg carrier rate in China noticeably (3). Additionally, “China Viral Hepatitis Prevention and Control Program (2017–2020)” and “National Action Plan for Eliminating Hepatitis C as a Public Health Threat (2021–2030)” were issued in 2017 and 2021, respectively (4). The National Reimbursement Drug List (NRDL) of healthcare insurance incorporated regular direct-acting antiviral agents (DAAs) (4). To deliver high-quality services, the WHO recommended providing hepatitis patients with accessible tests and treatments through decentralization of care to lower-level facilities at this stage (5).
To standardize the management of patients with chronic hepatitis and reduce the morbidity and mortality of hepatitis-related diseases, the community-based healthcare management pilot called “Love Deliver” was initiated in 2012 in Shanghai. Based on reported information from the Nationally Notifiable Disease Report System (NNDRS), community physicians provided service packages of 4 categories and 10 measures, including epidemiological investigation, disinfection, health education, longtime follow-up, free testing, and immunization to family caregivers who had given informed consent. In 2019, with the achievement of national health insurance negotiation and Volume-based procurement (VBP) both causing a significant reduction in drug prices of hepatitis, the expansion of basic medical care comprising hepatitis-related testing, referral, extended dispensing, and treatment in the community health centers (CHCs) was added to the pilot service package (Figure 1). By the end of 2023, 7,714 chronic hepatitis patients were contracted in the real-world study. This study aimed to evaluate the effectiveness of community-based healthcare management for chronic hepatitis patients, with public health and clinical care integration, and to provide a basis for the development of public health service strategies for chronic hepatitis B and C eliminations.
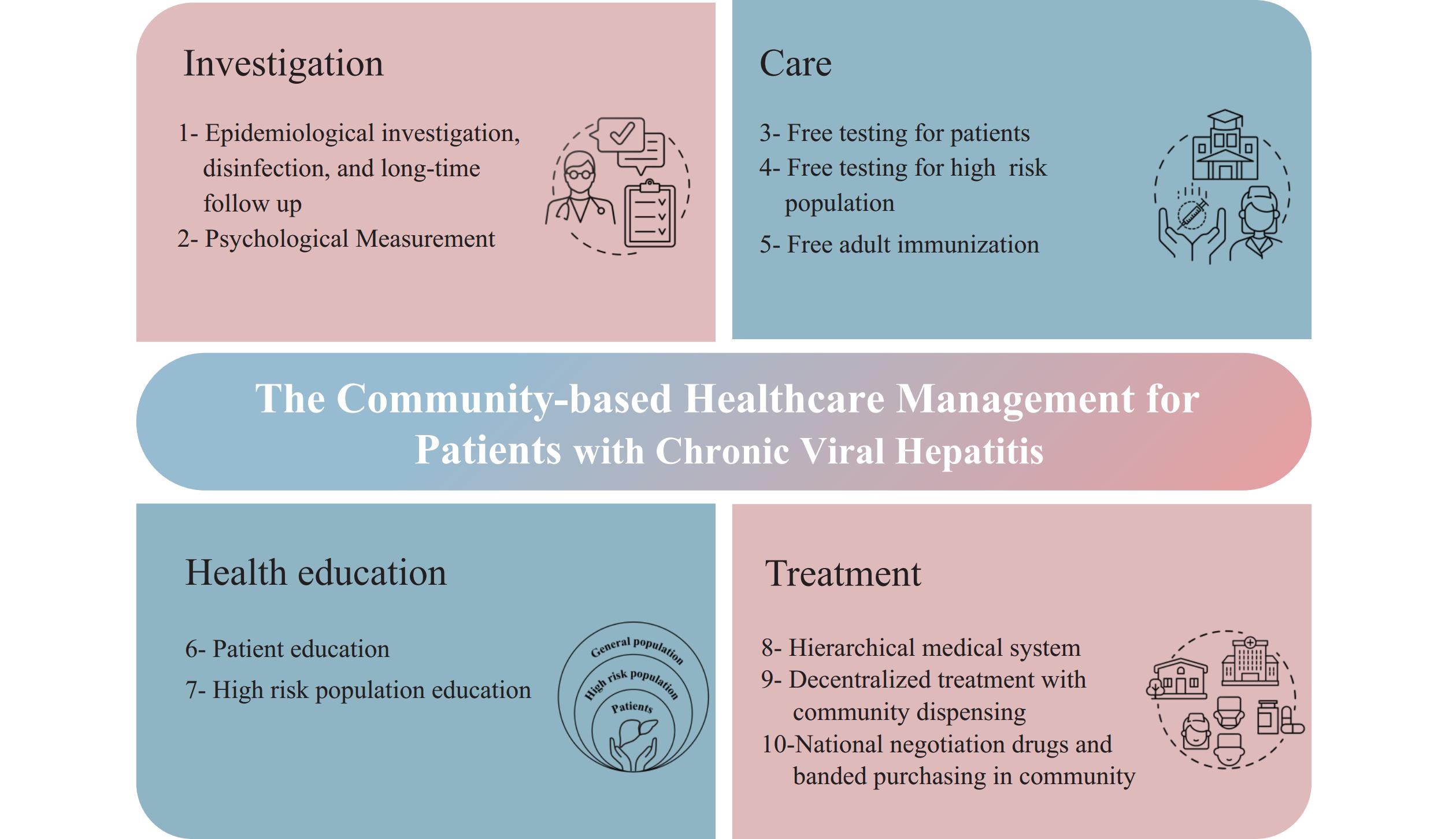 Figure 1.
Figure 1.The concrete measures of community-based healthcare management, 2012–2023.
Note: The community-based healthcare management for patients with chronic viral hepatitis comprised 10 measures in 4 categories. Measures 1–7 were executed over the past decade, and measures 8 to 10 of “Treatment” were added to the pilot service package between 2019 and 2023. Since 2020, the addition of first-line antiviral medications for hepatitis B patients and DAAs for hepatitis C patients to the list of medicines covered by the national medical insurance system has significantly improved their accessibility and affordability.Data was obtained from three cross-sectional surveys of a real-world study conducted in Shanghai, where patients could join or withdraw according to their preferences (Figure 2). In 2012, viral hepatitis patients from 30 communities were randomly selected from 5 central urban districts and 5 suburban districts using a multi-stage cluster sampling method, which served as the baseline as described in the “Study of disease burden of chronic hepatitis B and C patients in Shanghai based on Bronfenbrenner’s ecological systems theory: a community-based survey” and explained in the Disclosure statement (6). For the 2019 and 2023 surveys, patient information was drawn entirely from the “Love Deliver” pilot follow-up database, with all community-managed patients enrolled. All three surveys used identical questionnaires covering demographics, health status, treatment information, and the utilization of antiviral drugs and Hepatitis B vaccination among caregivers. The age-male-ALBI-platelets (aMAP) score was identified as the most prospective of all hepatocellular carcinoma (HCC) prediction models (7). The database was established using the EpiData Association (version 3.1 for Windows; Odense, Denmark), with descriptive statistical analysis and chi-square tests performed using SPSS Statistics (version 29.0 for Windows; IBM, Armonk, US). All statistical tests were two-sided, with a P value of <0.05 considered statistically significant.
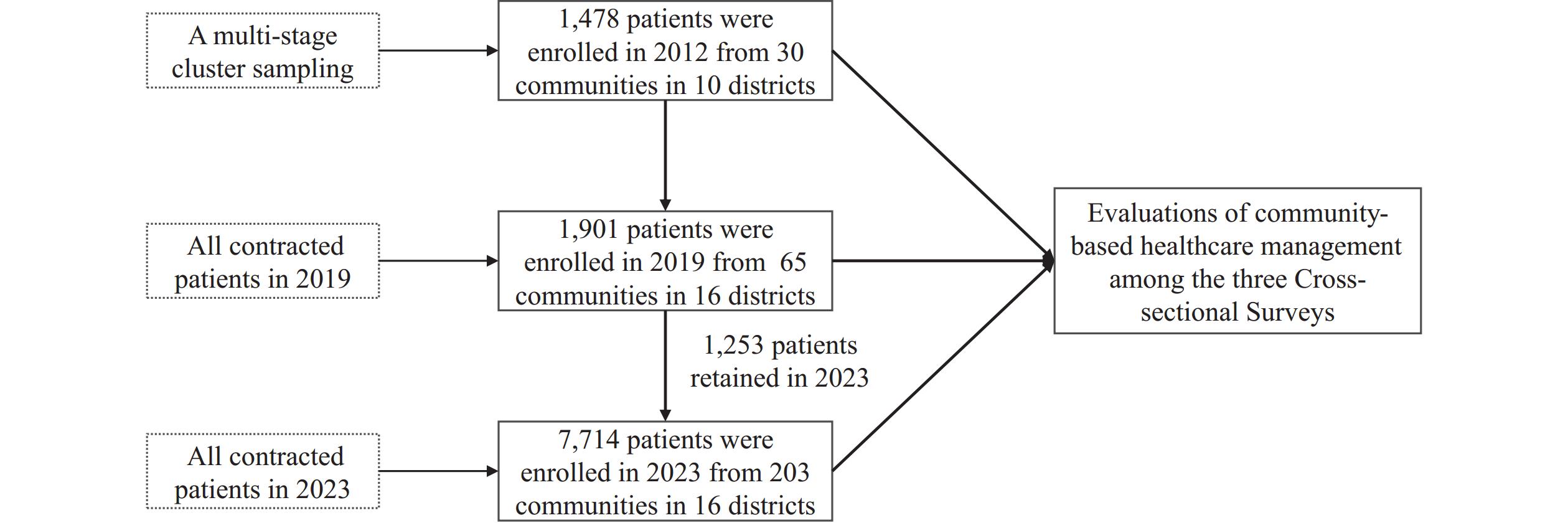 Figure 2.
Figure 2.Flow chart and sampling methodology for three cross-sectional surveys on the community-based healthcare management.
In this study, 1,478, 1,901, and 7,714 patients were recruited in 2012, 2019, and 2023, respectively. The mean age of patients across the three survey points increased progressively from 51.5±13.3 years in 2012 to 57.2±12.2 years in 2019, and 58.7±13.4 years in 2023 (Table 1).
Variables 2012, n (%) 2019, n (%) 2023, n (%) χ2 P Overall 1,478 1,901 7,714 Gender 17.376 <0.001 Male 951 (64.3) 1,155 (60.8) 3,935 (51.0) Female 527 (35.7) 746 (39.2) 3,779 (49.0) Age, years/mean±SD 51.5±13.3 57.2±12.2 58.7±13.4 21.558 <0.001 <40 314 (21.2) 195 (10.3) 891 (11.6) 40–49 283 (19.1) 288 (15.1) 1,095 (14.2) 50–59 493 (33.4) 512 (26.9) 1,584 (20.5) ≥60 388 (26.3) 906 (47.7) 4,144 (53.7) HBV DNA† 199.374 <0.001 Positive 233 (53.3) 82 (25.5) 454 (20.8) Negative 204 (46.7) 240 (74.5) 1729 (79.2) anti-HCV† 262.614 <0.001 Positive 78 (15.3) 65 (13.3) 558 (25.6) Negative 432 (84.7) 424 (86.7) 1,622 (74.4) Alanine aminotransferase (ALT)† 583.812 <0.001 Positive 413 (27.9) 166 (10.7) 436 (6.6) Negative 1,065 (72.1) 1,385 (89.3) 6,170 (93.4) Total Bilirubin (TBIL)† 150.485 <0.001 Normality 834 (85.6) 1,477 (94.3) 5,136 (95.1) Mild jaundice 125 (12.9) 87 (5.5) 252 (4.7) Moderate jaundice 13 (1.3) 1 (0.1) 9 (0.2) Severe jaundice 2 (0.2) 1 (0.1) 1 (0.0) Liver Ultrasound† 548.594 <0.001 Normality 904 (61.2) 975 (82.0) 3,634 (84.9) Mild fibrosis 534 (36.1) 136 (11.4) 477 (11.2) Severe fibrosis 28 (1.9) 40 (3.4) 108 (2.5) Nodular changes 12 (0.8) 38 (3.2) 60 (1.4) Hepatitis B vaccination of caregivers 610.153 <0.001 Full vaccination 369 (25.0) 535 (28.1) 3,820 (49.5) Partial vaccination 753 (50.9) 587 (30.9) 1,943 (25.2) Unvaccinated 312 (21.1) 615 (32.4) 1,951 (25.3) Unknow 44 (3.0) 164 (8.6) 0 (0) Treatment 655.103 <0.001 Yes 362 (24.5) 1,226 (64.5) 4,486 (58.2) No 1,116 (75.5) 675 (35.5) 3,228 (41.8) Community dispensing services* 11.418 <0.001 Prescriptions dispensed in CHCs / 173 (14.1) 508 (11.3) Others / 0 (0) 310 (6.9) No / 1,053 (85.9) 3,668 (81.8) aMAP score† 10.146 0.006 Low risk / 142 (25.0) 1,371 (25.4) Medium risk / 243 (42.8) 2,610 (48.3) High risk / 183 (32.2) 1,418 (26.3) Abbreviation: aMAP=age-male-ALBI-platelets; CHCs=community health centers; SD=standard deviation.
* Indicates the proportion of patients receiving various “community dispensing services” among those on antiviral therapy.
† Refers to patients who received relevant laboratory testing as part of management services.Table 1. Comparing the demographics and laboratory test results of chronic hepatitis patients in 2012, 2019, and 2023.
Among the 7,714 patients followed in 2023, 6,956 had chronic hepatitis, 32 had cirrhosis, and 16 had HCC, indicating a significant decline in cirrhosis and HCC rates compared to 2012 (ratio of 124.7∶8.6∶1.0). Antiviral treatment rates increased substantially from a baseline of 24.5% in 2012 to 64.5% in 2019 and 58.2% in 2023. Concurrently, abnormality rates for HBV DNA, ALT, TBIL, and fibrosis indices decreased significantly in 2019 and 2023. The 2023 aMAP scores revealed fewer individuals in high-risk HCC groups compared to 2019. Following the implementation of community dispensing services in 2019, 173 patients (14.1%, 173/1,226) in 2019 and 818 patients (18.2%, 818/4,486) in 2023 utilized these services. Additionally, hepatitis B vaccination rates among family members of hepatitis B patients increased compared to baseline levels (Table 1).
Furthermore, 1,253 patients managed in 2019 continued in the program through 2023. These patients showed significant improvements in clinical parameters, including reduced abnormality rates for HBV DNA (11.2% vs. 25.5%), ALT (3.4% vs. 10.7%), TBIL (2.8% vs. 5.7%), and fibrosis indices (15.3% vs. 18.0%), as well as higher rates of hepatitis B vaccination and community dispensing service utilization after five years of follow-up care. Analysis of patients lost to follow-up revealed that 10% (66/648) had died between 2019 and 2023 according to the Vital Statistics System. Additionally, 63% (410/648) of surviving patients who were lost to follow-up primarily resided in urban areas and may have relocated to their hometowns due to the coronavirus disease 2019 (COVID-19) pandemic.
The study consistently demonstrated the therapeutic effectiveness of antiviral therapy across all three surveys. Using the 2023 results as a reference, treated patients showed higher HBeAg (χ2=33.996, P<0.05) and HCV RNA (χ2=20.664, P<0.05) positivity rates compared to untreated patients. Conversely, HBV DNA positivity rates were significantly lower in treated patients (χ2=19.924, P<0.05). The aMAP scores indicated that treated patients had a lower proportion of individuals at high risk for HCC (χ2=13.982, P<0.05). Overall, aMAP scores revealed that 1,899 (24.6%) of managed patients remained at high risk for HCC, highlighting the importance of continued HCC surveillance and treatment services for this population.
-
With the continuous refinement of the pilot strategy and gradual expansion of services, the community-based healthcare management package has been delivered to 7,714 patients across 203 communities in all 16 districts of Shanghai by 2023. Our findings indicate that antiviral treatment rates among contracted patients in 2019 and 2023 increased significantly from baseline levels. However, a slight decline was observed as the number of contracted patients expanded to approximately four times that of 2019. Additionally, in 2023, community physicians received enhanced training on the Guidelines for the Prevention and Treatment of Chronic Hepatitis B (version 2022), improving their knowledge of antiviral therapy drugs compared to 2019 (8). This may have led community physicians to exclude patients taking hepatoprotective drugs or other non-antiviral medications. Despite a twelve-year aging trend among patients (from 2012 to 2023), their clinical conditions improved. For instance, abnormality rates in laboratory indices including HBV DNA, ALT, TBIL, and fibrosis were reduced. The likelihood of developing HCC, as indicated by aMAP scores, also decreased. These results were consistent with outcomes observed in the 1,253 patients followed in both 2019 and 2023, suggesting that long-term management can increase treatment rates and lead to effective clinical improvements for contracted patients.
In contrast to baseline (2012), when antiviral treatment and testing services were primarily provided by specialists, a significant portion of contracted patients accepted prescriptions from community general practitioners after project implementation. Community dispensing, which provided high reimbursement rates, demonstrated that decentralization can be effectively aligned with broader healthcare strategies. Moreover, free adult vaccination rates for caregivers were significantly higher than at baseline, demonstrating that community-contracted management can enhance adult immunization and basic public health services for caregivers. In the Global Hepatitis Report 2024, WHO proposed four strategic directions and ten actions to advance a public health approach in low and middle-income countries (9) with a key direction being the delivery of high-quality, evidence-based, people-centered services. The healthcare management pilot in Shanghai, which decentralized management of patients to community health centers, provided patients with extended prescriptions, accessible and affordable drugs, HCV RNA testing, and free hepatitis B vaccination for caregivers. This community-based health services package effectively responds to the WHO’s decentralization strategy (5).
Compared to untreated patients, those receiving antiviral therapy in the community demonstrated lower HBV-DNA positivity rates and reduced risk of developing HCC, particularly among males under 60 years of age. Previous clinical studies have confirmed that antiviral therapy effectively delays liver fibrosis progression and reduces HCC incidence in patients with HBV and HCV (10), findings that our study further validates. Additionally, patients who do not currently meet treatment criteria should be monitored annually for disease progression, ALT levels, and HBV DNA levels as recommended by clinical guidelines. Timely intervention should be provided for patients who have previously deferred treatment (11).
Several limitations of this study should be acknowledged. First, this research was conducted in a real-world setting where patients could freely enter or exit the management program in pursuit of higher-quality services. Natural mortality, excess deaths, and relocation (60% of lost patients were not local residents) due to the COVID-19 pandemic resulted in approximately 34% attrition between 2019 and 2023. While survivor bias likely exists, its magnitude is difficult to estimate. Second, although laboratory results were obtained using standardized thresholds established by secondary and tertiary medical institutions, minor variations may have occurred depending on test reagent brands and methodologies. Third, the quasi-ecological design of this study makes it difficult to determine whether the service packages played a decisive role compared to the broader medical environment. In the next phase, our research team plans to compare disease progression, complication incidence, treatment outcomes, and quality of life between managed and unmanaged patients reported in NNDRS to more accurately evaluate the effectiveness of community health management services.
In conclusion, the community-based healthcare management pilot for patients with chronic viral hepatitis — which includes community dispensing, nationally negotiated drugs, volume-based procurement at the community level, hepatitis-related testing, and free hepatitis B vaccination for adults — provides a reference model for establishing decentralized testing, care, and treatment approaches for chronic viral hepatitis patients. This model effectively combines access to care and treatment with a comprehensive public health approach.
-
This study shared part of the results from 2012 with another study published in Chinese entitled “Study of disease burden of chronic hepatitis B and C patients in Shanghai based on Bronfenbrenner’s ecological systems theory:a community — based survey” authored by Dr. Hong Ren. That study attempted to establish a novel public health strategy for chronic hepatitis patients based on community engagement and communication between medical institutions and public health departments. In contrast, the current study evaluated the effectiveness of a community-based healthcare management model for chronic hepatitis patients between 2012 and 2023, with an extension of basic medical care added in 2019 that comprised hepatitis-related testing, referral, extended dispensing, and treatment in the community.
-
This study collected data based on home visit records from family doctors. We thank all participants who contributed to the study by collecting and sharing data.
-
Approved by the Ethics Review Committee of Shanghai Center for Disease Control and Prevention (No.: 2016-21 and 2022-39) and complied with the relevant statements of the Declaration of Helsinki.
HTML
| Citation: |



 Download:
Download:




