-
Objective: Fleas are vectors for the transmission of various pathogens. The study is to understand the pathogens carried by parasitic fleas in domestic dogs and to evaluate the pathogenic potential risk to humans.
Methods: 121 fleas were collected from 6 dogs in different farmers’ households in Suichuan County, Ji’an City, Jiangxi Province in July 2023. Flea species were determined through morphological identification and CoII gene detection. Whole genomic DNA was extracted from all 121 fleas, and six pathogens - Borrelia burgdorferi, Borrelia miyamotoi, Anaplasma phagocytophilum, Coxiella burnetii, spotted fever group Rickettsia, and Ehrlichia chaffiensis — were detected using nested polymerase chain reaction (PCR).
Results: Positive products were sequenced, and the carrier status of each pathogen was analyzed. Of the 121 fleas identified, 118 were Pulex irritans and 3 were Ctenocephalides felis. PCR results revealed that Borrelia burgdorferi (5%, 6/118), Borrelia miyamotoi (0.8%, 1/118), Borrelia hermsii (9%, 11/118), Anaplasma phagocytophilum (0.8%, 1/118), spotted fever group Rickettsia (0.8%, 1/118), and Coxiella burnetii (0.8%, 1/118) were detected in Pulex irritans. Additionally, one sample showed mixed infection with both Borrelia burgdorferi and Anaplasma phagocytophilum (0.8%, 1/118).
Conclusions: This study suggests that Pulex irritans can carry multiple pathogens, with implications for public health needs that warrant further investigation.
-
Fleas are ectoparasites that infest humans and animals, capable of carrying and transmitting various pathogens through bites. Species like Pulex irritans, Xenopsylla cheopis, and Ctenocephalides felis are known vectors of zoonotic diseases such as plague, murine typhus, and spotted fever (1). Flea-borne diseases pose significant risks to human and animal health, with their prevalence influenced by environmental factors like temperature changes.
Jiangxi Province, located in Southeast China, has a warm and humid climate ideal for the breeding of medical insects, including fleas. While studies have examined flea distribution and pathogen carriage in some regions like Yunnan Province and Inner Mongolia Autonomous Region, contributing to understanding local flea-borne diseases (2–3), research on flea-borne pathogens in Jiangxi remains limited.
Understanding the pathogens carried by fleas in Jiangxi Province is crucial for controlling flea-borne diseases. In July 2023, 121 fleas were collected from six dogs in Suichuan County, Ji’an City, Jiangxi Province. Six pathogens, including Borrelia burgdorferi (B. burgdorferi), Borrelia miyamotoi (B. miyamotoi), Anaplasma phagocytophilum (A. phagocytophilum), Coxiella burnetii (C. burnetii), spotted fever group Rickettsia (SFGR), and Ehrlichia chaffiensis (EC) were detected via nested PCR and sequencing. These findings provide essential data for local disease surveillance and control. The CoII gene, with primers F-leu: TCTAATATGGCAGATTAGTGC and R-lys: GAGACAGTACTTGCTTTCAGTCATC (4), was used for flea species identification.
Six pathogens, including B. burgdorferi, B. miyamotoi, A. phagocytophilum, C. burnetii, SFGR, and EC were detected via nested polymerase chain reaction (PCR) method. The sequences of target genes and primers for PCR detection are shown in Table 1 (5–7). Amplified products were electrophoresed on a 1.5% agarose gel, and positive samples were purified, sequenced by Beijing De Aoping Biotechnology Co., Ltd., and analyzed using NCBI BLAST for homology comparison. Reference sequences from GenBank were used to construct a phylogenetic tree with MEGA11.0. Additionally, B. burgdorferi and Borrelia hermsii (B. hermsii) positive samples were tested via quantitative real-time PCR (qPCR) targeting the recA and fla-B genes, respectively, using a Probe qPCR mix (Premix Ex TaqTM, TaKaRa) on a LightCycler 480 System (Roche Diagnostics, United States).
Bacteria Target gene Primer name Sequence (5’–3’) Size (bp) Reference
Borrelia burgdorferi5S-23S rRNA
IGSP1 CGACCTTCTTCGCCTTAAAGC 255
(5)P2 TAAGCTGACTAATACTAATTACCC P3 TCCTAGGCATTCACCATA P4 GAGTTCGCGGGAGA Borrelia miyamotoi glpQ Q1 CACCATTGATCATAGCTCACAG 424 Q2 CTGTTGGTGCTTCATTCCAGTC Q3 GCTAGTGGGTATCTTCCAGAAC Q4 CTTGTTCTTTATGCCAGAAGGGT Anaplasma phagocytophilum 16S rRNA AP-F GTCGAACGGATTATTCTTTATAGCTTG 389 AP-R TATAGGTACCGTCATTATCTTCCCTAC Ehrlichia chaffiensis 16S rDNA ECB AGAACGAACGCTGGCGGCAAGCC 389 ECC CGTATTACCGCGGCTGCTGGCA H3 TATAGGTACCGTCATTATCTTCCCTAT H1 CAATTGCTTATAACCTTTTGGTTATAAAT Spotted fever group Rickettsia ompA F ATGGCGAATATTTCTCCAAAA 533 R GTTCCGTTAATGGCAGCATCT 602R AGTGCAGCATTGGCTCCCCCT Coxiella burnetii IS1111 F1 TACTGGGTCTTGATATTGC 297 R1 CCGTTTCATCCGCGGTG F2 GTAAAGTGATCTACACGA R2 TTAACAGCGCTTGAACGT Borrelia burgdorferi recA F AGGTGGGATAGCTGCTTTTATTGAT 83
(6)R GTTCTGCAACATTAACACCTAAAGCTT P 6-FAM-ACAGGATCAAGAGCATG-BHQ1 Borrelia hermsii fla-B F GGACATTGAGAGTACATGTGGGC 136 (7) R CCTCTTGCTGTCCTATCTCTTGCA P AGCCTGAGCRCCTTCACCTGCAAAAAGA Table 1. Sequences of target genes and primers for polymerase chain reaction detection.
The 121 collected fleas were disinfected by soaking in sodium hypochlorite bleach for 30 seconds, followed by immersion in 75% alcohol for 10 minutes, washed twice, and rinsed with ultra-pure water. Each flea was placed in a 1.5 mL grinding tube and homogenized using a high-throughput grinder. Morphological characteristics and CoII gene detection and sequencing identified 3 Ctenocephalides felis and 118 Pulex irritans. The sequence analysis of the flea CoII gene is shown in Figure 1.
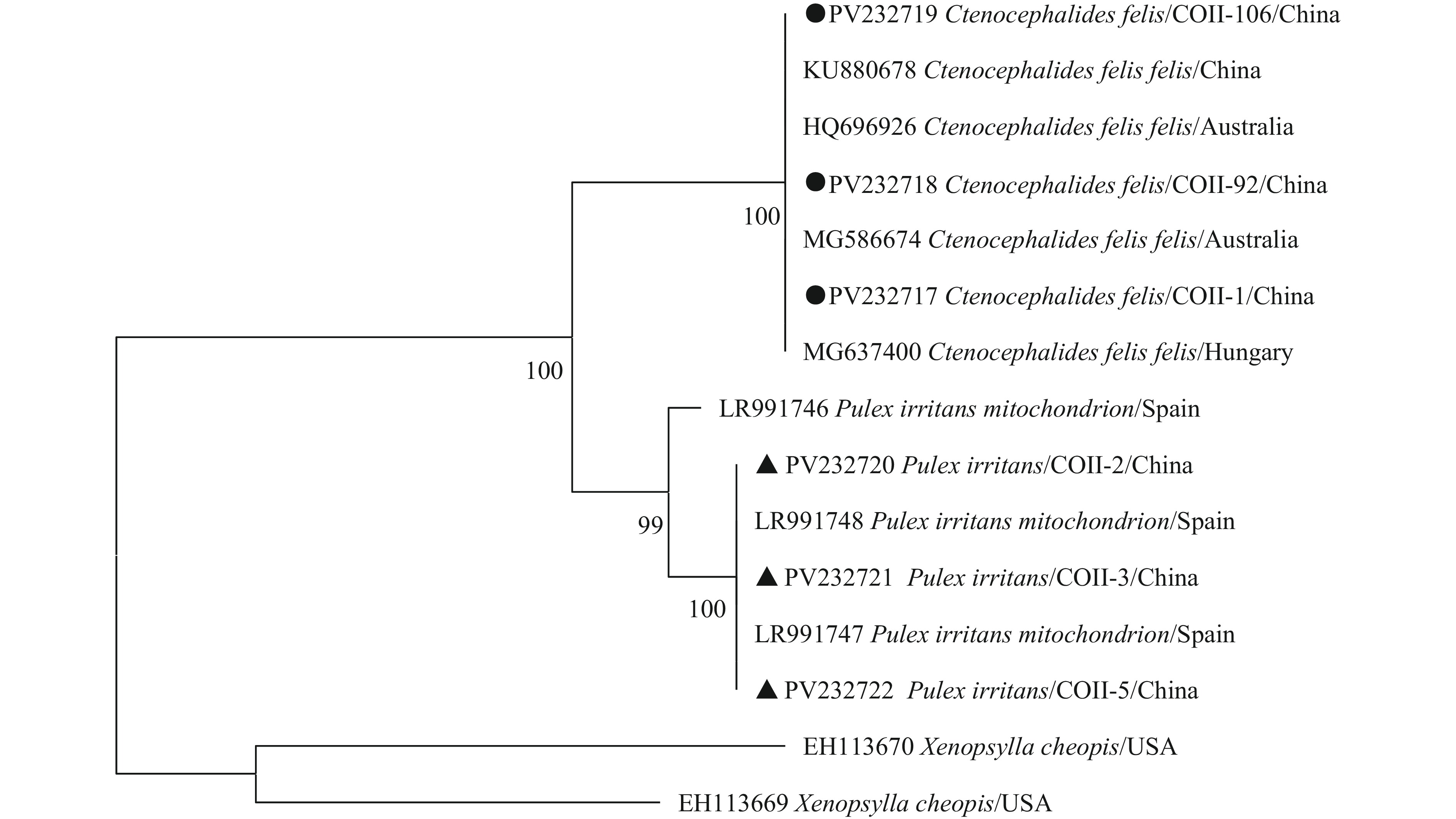 Figure 1.
Figure 1.COII sequence analysis of fleas in Ji ’an city, Jiangxi Province, China.
Note: ● Means flea samples identified as Ctenocephalides felis in this study; ▲ Means flea samples identified as Pulex irritans in this study.Pathogen detection in 118 Pulex irritans revealed B. burgdorferi (5%, 6/118), B. miyamotoi (0.8%, 1/118), B. hermsii (9%, 11/118), A. phagocytophilum (0.8%, 1/118), Rickettsia slovaca (0.8%, 1/118), and C. burnetii (0.8%, 1/118). One sample showed mixed infection with B. burgdorferi and A. phagocytophilum. E. chaffeensis was negative, and no pathogens were detected in the 3 Ctenocephalides felis.
In this study, six positive samples for B. burgdorferi were sequenced and analyzed (Figure 2A). The results revealed that the rrf–rrl spacer sequences from samples Bb-3, Bb-34, and Bb-109 were identical to that of Borrelia garinii (B. garinii), while the rrf–rrl spacer sequence from sample Bb-5 was identical to that of Borrelia burgdorferi sensu stricto (B. b. s. s.). Additionally, the rrf–rrl spacer sequences from samples Bb-52 and Bb-91 were identical to that of Borrelia valaisiana (B. valaisiana). Based on the nested PCR results, the six positive samples for B. burgdorferi were also tested via qPCR, which confirmed that samples Bb-3, Bb-5 , and Bb-109 were positive for the B.burgdorferi recA gene.
 Figure 2.
Figure 2.Phylogenetic analysis of six pathogens in Pulex irritans.(A) Phylogenetic analysis for 5S-23S rRNA gene of Borrelia burgdorferi; (B) Phylogenetic analysis for glpQ gene of Borrelia miyamotoi; (C) Phylogenetic analysis for glpQ gene of Borrelia hermsii; (D) Phylogenetic analysis for 16S rRNA gene of Anaplasma phagocytophilum; (E) Phylogenetic analysis for IS1111 gene of Coxiella burnetii; (F) Phylogenetic analysis for ompA gene of spotted fever group Rickettsia.
Note: for (A), the sequences clustered as Borrelia garinii were marked as "●"; Borrelia burgdorferi sensu stricto were marked as "▲"; Borrelia valaisiana were marked as "◆"; for (B), the sequences clustered as Borrelia miyamotoi were marked as "●"; for (C), The sequences identified as Borrelia hermsii are marked with "●"; for (D), The sequence identified as Anaplasma phagocytophilum is marked with "●"; for (E), The sequences clustered as Coxiella burnetii were marked as "●"; for (F), The sequences clustered as Rickettsia slovaca were marked as "●".A total of 11 positive samples for B. hermsii and 1 positive sample for B. miyamotoi were sequenced and analyzed (Figure 2B–C). After removing duplicate sequences, the remaining 8 unique sequences were used to construct the phylogenetic tree of B. hermsii. These 8 sequences were found to be closely related to the USA strain LAK-6 (KX171818). The glpQ sequence of B. miyamotoi detected in sample Mi-112 was closely related to USA clone GlpQ52T4 (KY008451). Based on the nested PCR results, the 11 samples positive for B. hermsii were also tested via qPCR, which confirmed that sample B.h-103 was positive for B. hermsii fla-B gene.
The phylogenetic analysis results forA. phagocytophilum, C. burnetii, and SFGR are shown in Figure 2D, 2F. The 16S rRNA sequence of A. phagocytophilum detected in sample AP-15 was closely related to China strain TMSK (PQ459373). The IS1111 sequence of C. burnetii detected in sample IS-6 was closely related to Mexico isolate INIFAP Cap04 insertion (MT459145). The ompA sequence of SFGR detected in sample SF-17 was closely related to Turkey isolate Ro-715 (MF379305), which is an isolate of Rickettsia slovaca.
-
Fleas are recognized as one of the most important vectors of diseases in humans and animals, with flea-borne diseases potentially re-emerging as epidemics due to environmental and behavioral changes affecting vector-host ecology (8). This study is the first to report Borrelia burgdorferi, Borrelia miyamotoi, Borrelia hermsii, Anaplasma phagocytophilum, Rickettsia slovaca, and Coxiella burnetii in Pulex irritans.
B. burgdorferi, the causative agent of Lyme disease, and a primary pathogen typically transmitted by ticks, was detected in Pulex irritans in Jiangxi Province. Among 20 Pulex irritans positive for flea-borne bacteria, three carried B. garinii, two carried B. valaisiana, and one carried B. b. s. s. Additionally, three of the six positive samples were confirmedpositive results for B. burgdorferi recA by qPCR.These findings indicate that Pulex irritans in Jiangxi may harbor three B. burgdorferi genotypes, with B. garinii being the predominant pathogenic genotype in China and B. b. s. s. being most common in the Americas (9). Notably, these genotypes have also been detected in ticks from Jiangxi Province (10).
B. miyamotoi and B. hermsii can cause tick-borne relapsing fever. While B. miyamotoi is transmitted by Ixodes ticks, B. hermsii is vectored by soft ticks. Through nucleic acid detection of the glpQ gene, 1 Pulex irritans sample was positive for B. miyamotoi, and 11 Pulex irritans samples were positive for B. hermsii. Furthermore, one of the 11 B. hermsii positive samples was confirmed positive for B. hermsii fla-B by qPCR. These results demonstrate that Pulex irritans can carry both B. hermsii and B. miyamotoi.
Nucleic acid detection revealed one Pulex irritans sample positive for A. phagocytophilum, one positive for SFGR, and one positive for C. burnetii. Previous studies in China have reported Ctenocephalides felis carrying C. burnetii and SFGR (11-12). In northwestern Iran, Pulex irritans has been documented to carry Rickettsia sp. (13). However, this is the first report of Pulex irritans carrying C. burnetii and Rickettsia slovaca.
Among the 118 Pulex irritans samples, one tested positive for both B. burgdorferi and A. phagocytophilum, indicating that a single flea can harbor multiple pathogens. This finding underscores the necessity for comprehensive pathogen detection in fleas.
A previous study reported the isolation of Borrelia yangzensis from rodents in six counties in Jiangxi Province (14). Additionally, tick-borne pathogens including B. burgdorferi, B. miyamotoi, and SFGR have been detected in patients with arthritis or neurological symptoms (15), indicating that Ji’an City is a natural focus for tick-borne diseases with potential for zoonotic transmission. While ticks are the primary vectors for B. burgdorferi, B. miyamotoi, B. hermsii, A. phagocytophilum, C. burnetii, SFGR, and E. chaffiensis, our study identifies Pulex irritans as a potential carrier of these pathogens, highlighting the need for further investigation into its public health significance in pathogen transmission.
Due to the lack of methods to verify the prevalence of these six pathogens in host dogs and considering the influence of sample size, collection points, and other factors on pathogen detection rates, long-term monitoring and investigation of flea-borne pathogens are necessary. Additionally, strengthening the development of control measures for flea-borne diseases in Jiangxi Province is essential.
-
Not involving ethics.
HTML
| Citation: |



 Download:
Download:




