-
Introduction: On August 3, 2024, a suspected case of Lassa fever was identified in Sichuan Province, likely imported from an endemic region. Local CDCs promptly initiated investigation and implemented preventive measures upon receiving this report.
Methods: The response included epidemiological investigations, contact tracing and management, hospital infection control measures, environmental disinfection, laboratory testing, biological sample management, and risk communication strategies.
Results: The patient was confirmed as China’s first imported case of Lassa fever on August 6. The investigation identified 12 close contacts and 71 general contacts. By August 24, all contacts had completed medical observation without developing any symptoms consistent with Lassa fever. The patient recovered with residual hearing loss and was discharged on September 24 following expert verification.
Conclusions: The increasing frequency of international travel and ongoing globalization have elevated the risk of novel infectious disease importation, including Lassa fever. Simultaneously, the widespread adoption of metagenomic sequencing for diagnosing unexplained illnesses in healthcare settings has enhanced pathogen detection sensitivity, enabling more precise identification of emerging infectious agents.
-
Lassa fever (LF) is an acute viral hemorrhagic illness caused by Lassa virus (LASV), which was first identified in 1969 in the Nigerian town of Lassa (1). The disease is primarily transmitted through contact with rodents, particularly Mastomys natalensis, which serves as the primary natural reservoir, along with black house mice and small chevrotains (2). Human infection occurs through direct contact with infected animals and their excreta, as well as exposure to blood, bodily fluids, or excretions from infected patients. Healthcare workers may become infected when treating LF patients without proper protective equipment, including during the handling of deceased patients. Additionally, laboratory-acquired infections can occur through improper biosafety practices. There is no documented evidence of airborne person-to-person transmission (3-6).
This report describes the first imported case of LF in China, from Guinea, and details the public health response measures implemented to prevent secondary transmission.
-
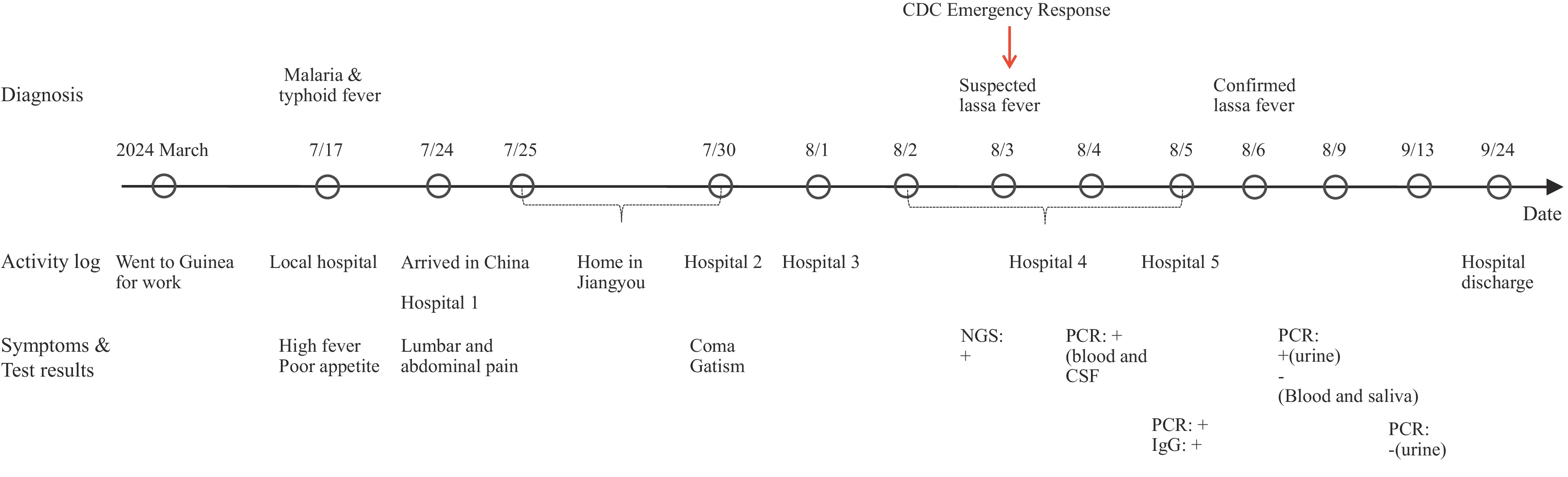
A 49-year-old female, employed as a cook in Guinea, Africa since March 2024, presented with lumbar pain, abdominal pain, frequent urination with urgency, nausea, vomiting, dry mouth, and general malaise upon returning to China on July 24. She subsequently sought medical evaluation at four different hospitals. The patient’s initial presenting symptoms included altered consciousness and unexplained fever. Following treatment, her vital signs stabilized, consciousness cleared, though she developed severe hearing impairment (near-complete deafness). While her reading and writing abilities remained intact, she exhibited scattered cutaneous hemorrhages and bruising at venipuncture sites. Laboratory findings revealed acute renal insufficiency and elevated transaminases, though cardiopulmonary function remained adequate. On August 5, the patient was transferred to a negative pressure isolation room at a designated infectious disease hospital.
During her stay in Guinea, the patient was stationed in Mamou, a region bordering Sierra Leone, where she worked as a camp cook at a factory. The patient reported unremarkable living conditions with no apparent rodent activity and no similar cases among local residents. On July 17, 2024, she developed high fever and anorexia while working in the Mamou Region. Local hospital evaluation yielded positive results for both Plasmodium via microscopic blood smear examination and typhoid fever via Widal test. Treatment with artemisinin and levofloxacin initially provided symptomatic relief.
The patient departed Guinea on July 23, 2024, and arrived in China on July 24 afternoon. She traveled by private vehicle to Mianyang City, Sichuan Province, arriving at the bus terminal that evening. After taking a taxi to the first hospital, she underwent medical evaluation and overnight observation. On July 25, she was discharged and returned to her apartment via private transport. She remained in isolation at home until July 30, when her daughter-in-law found her collapsed and incontinent. The patient was subsequently transported to the second hospital in Jiangyou City. On August 1 and 2, she was transferred via ambulance to the third hospital in Mianyan City and the fourth hospital in Chengdu City, respectively. Cerebrospinal fluid and blood samples were collected on August 3 and 4. On August 5, she was transferred to the ICU negative-pressure isolation ward at the fifth hospital (infectious disease hospital) for treatment. By September 24, the patient had recovered except for persistent hearing loss and was discharged following expert verification. The complete epidemiological timeline is illustrated in Figure 1.
-
Initial blood samples tested negative for Plasmodium at both Jiangyou CDC and Sichuan CDC on August 1 and 2, 2024, respectively. On August 2, cerebrospinal fluid collected at the third hospital was analyzed using targeted next-generation sequencing (tNGS) at a commercial medical laboratory, which yielded positive detection of LASV. Subsequent fluorescence quantitative Polymerase Chain Reaction (PCR) testing by Sichuan CDC confirmed positive results in both cerebrospinal fluid and blood samples. China CDC conducted comprehensive testing on 5 non-inactivated samples (2 cerebrospinal fluid and 3 blood), with all samples testing positive by fluorescence quantitative PCR and all 3 blood samples showing positive IgG antibodies. Fluorescence quantitative PCR analysis for Salmonella typhi, Salmonella paratyphi and Salmonella yielded negative results. On August 6, following expert consultation and review of epidemiological characteristics, clinical presentation, and laboratory findings confirming both LASV nucleic acid and IgG antibodies, the case was definitively diagnosed as severe LF. Post-treatment monitoring showed negative PCR results for blood and saliva samples, though urine samples remained positive for approximately one month before converting to negative prior to hospital discharge. Whole genome sequencing performed independently by China CDC and Sichuan provincial CDC on cerebrospinal fluid and urine samples revealed LASV genotype IV, consistent with known geographical and epidemiological distribution patterns. Detailed results and specific sampling time are shown in Table 1.
Samples Plasmodium by microscopic examination Salmonella typhi, Salmonella paratyphi and Salmonella by fluorescence quantitative PCR LASV by fluorescence quantitative PCR Blood by LASV IgG antibody LASV Genetic sequence Blood Blood CSF Blood Urine Saliva Aug,1 Negative* − − (+) 31.45§ − − (+)§ Aug,2 Negative* − (+) 34.92*, 30.79§ − − − − (+)† Aug,3 − − (+) 32.89§ (+) 36.66*, 35.28§ − − (+)§ (+)§ Clade Ⅳ Aug,4 − − − (+) 37.18§ − − (+)§ − Aug,8 − Negative¶ − Negative* − − − − Aug,9 − − − Negative* (+) 33.36* Negative* − − Aug,12 − − − Negative* (+) 31.62* Negative* − − Aug,16 − − − − (+) 31.52* − − − Aug,31 − − − − (+) 33.22* − − − Sept,10 − − − − (+) 36.57* − − − Sept,13 − − − − Negative* − − − Sept,17 − − − − Negative* − − − Note: “−” means no sample collected; “+” means positive.
Abbreviation: LASV=Lassa virus; CSF=Cerebrospinal fluid.
* Sichuan Provincial CDC.
† Third-party testing company.
§ China CDC.
¶ Chengdu CDC.Table 1. Laboratory test results of biological samples from the patient.
-
Close contacts were defined as: 1) individuals who lived or worked with the patient; 2) personnel involved in medical diagnosis, treatment (invasive or non-invasive), caregiving, laboratory testing, and sample handling who may have had direct contact with the patient’s blood, bloody secretions, excreta, or contaminated materials without taking standard protective measures. General contacts were defined as individuals who had contact with the patient outside these categories and did not take standard protective measures. The investigation identified 12 close contacts and 71 general contacts. All contacts underwent temperature monitoring and symptom screening twice a day for 21 days following their last exposure. Three general contacts developed fever symptoms, while all other contacts remained asymptomatic. Blood, saliva, and urine samples were collected from all 12 close contacts both at initial management and at the end of the medical observation period. Blood samples were also collected from the three general contacts with fever. All samples tested negative for LASV by PCR analysis. By August 24, all contacts had completed their medical observation period without developing any suspected clinical symptoms of LF.
Additional public health measures implemented by CDCs included: 1) enhanced infection control protocols at all medical facilities where the patient received treatment; 2) comprehensive environmental disinfection of potentially contaminated areas; 3) stringent management of clinical specimens; and 4) targeted health education about LF for the patient, family members, medical staff, and other contacts.
-
In accordance with the International Health Regulations (IHR), China’s national focal point officially reported the case to the World Health Organization (WHO). Following WHO’s request, supplementary epidemiological investigation details regarding the patient’s residence in Guinea were provided. However, since the airline had already departed when the case was identified, further investigation of potential exposure among flight passengers and crew members could not be conducted.
-
Research indicates that LF exhibits significant regional variation in case fatality rates across West Africa, ranging from 16.5% to 50.0%. An estimated 300,000 new infections and 5,000 deaths occur annually throughout Western Africa, with recent Nigerian outbreaks reporting fatality rates of up to 25.4% (7). Cases exported from Africa have been documented in various countries and regions, with some instances resulting in secondary transmission (3-4). The disease demonstrates particularly high mortality among symptomatic cases and poses severe risks to pregnant women and fetuses. The WHO has designated LF as a priority disease for global research and development. Given the strengthening ties between China and Africa, the risk of LF importation into China is likely to increase, necessitating proactive preparedness measures.
This imported case of LF was effectively contained, and no local transmission occurred. This conclusion is supported by the following factors. First, LF is endemic to West Africa, including Guinea, with no previous cases detected in China. The patient’s work history in Guinea from March to July 2024, coupled with symptom onset prior to entering China, indicates infection occurred during her stay in Guinea. Second, the primary reservoir host, Mastomys species, has not been documented in China. Human-to-human transmission typically occurs through contact with infected bodily fluids, with secondary cases predominantly occurring among caregivers, healthcare workers, and those handling deceased patients. Among the case’s contacts, only three close contacts developed fever due to other illnesses, with no link to LF. All 83 contacts completed 21-day observation periods, demonstrating neither clinical signs of LF nor positive results on reverse transcription-polymerase chain reaction (RT-PCR) testing. Third, immediate implementation of comprehensive control measures — including patient isolation, contact tracing, medical observation, and environmental disinfection — effectively contained any potential spread. The patient was isolated and medically monitored until testing negative in blood, saliva, and urine.
Looking ahead, China faces increased risk of emerging infectious disease importation, including LF, particularly in major port cities due to international travel and commerce. This necessitates several strategic improvements: enhanced entry quarantine and disease screening capabilities in areas with significant international traffic; improved health declaration awareness among travelers; strengthened clinical vigilance for emerging infections in healthcare settings, including thorough travel history documentation; and expanded laboratory capacity for LF testing at all levels of disease control organizations. When feasible, implementation of advanced technologies such as multi-pathogen detection and metagenomic sequencing can facilitate rapid identification of unknown pathogens. Enhanced diagnostic capabilities could significantly improve clinical management and prevention of LF in at-risk populations (8). While no approved vaccines or therapeutics currently exist for LF, several promising candidates are undergoing clinical trials (9). Accelerated vaccine development and commercialization efforts remain crucial. For individuals traveling to or working in Africa, comprehensive education about LF prevention is essential, emphasizing rodent avoidance, food protection, and strict hygiene practices. Organizations should provide targeted health education regarding locally prevalent infectious diseases to Chinese residents abroad.
-
Received ethical approval from the Ethics Committee of the Chengdu Center for Disease Control and Prevention, China (approval number: 2025018).
HTML
Case Report
Laboratory Testing
Contact Tracing and Infection Control Measures
International Alert
| Citation: |



 Download:
Download:




