HTML
-
Introduction: Vaccination stands as the most effective preventive measure against yellow fever (YF). However, the YF vaccination associated Adverse Events following Immunization (AEFI) cases occur occasionally.
Methods: The Guangdong Provincial Center for Disease Control and Prevention utilized Targeted Next-Generation Sequencing (tNGS) to determine whether the imported suspected YF case was infected by the wild-type YF virus strain or experienced an AEFI.
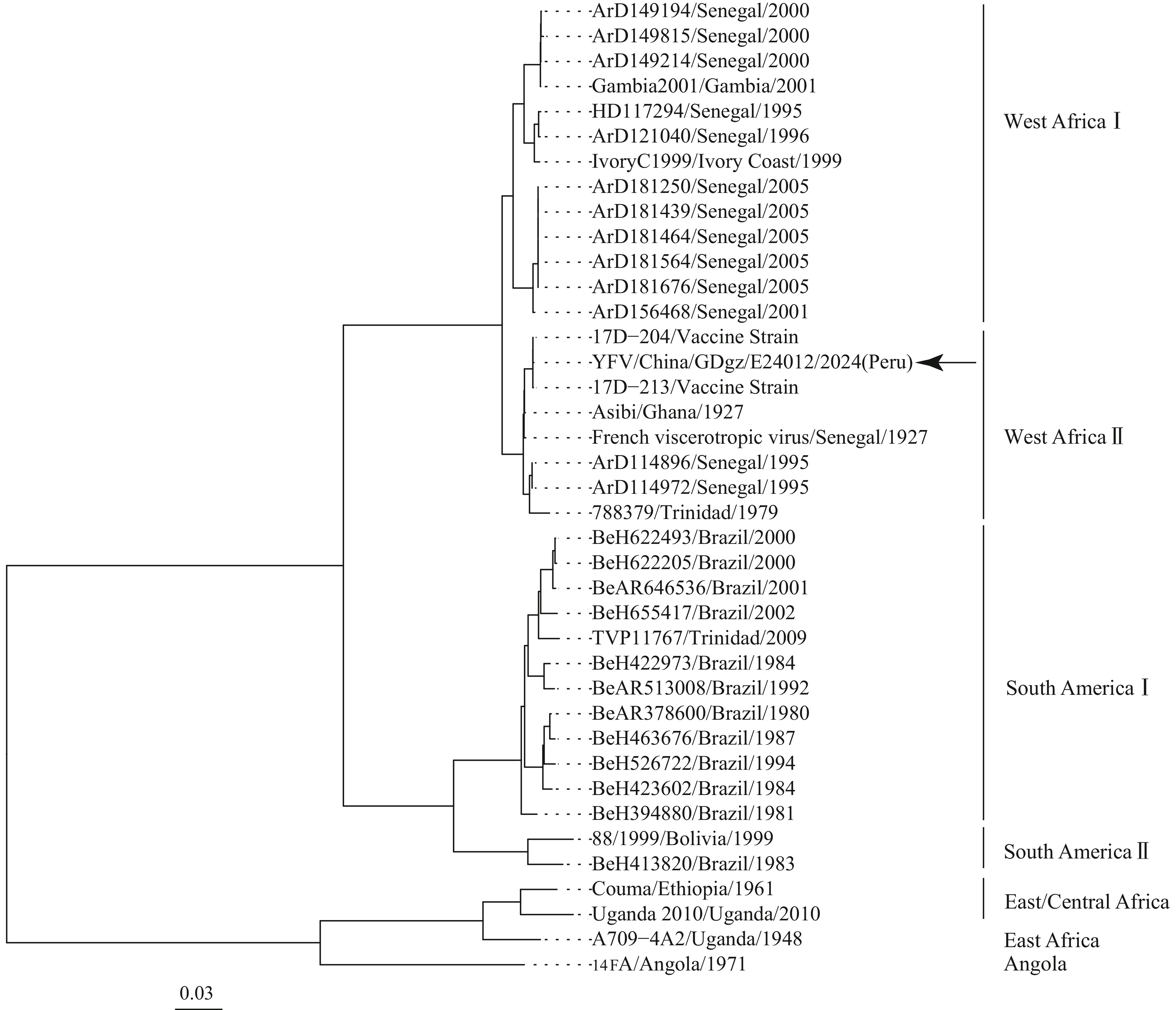
Results: tNGS analysis successfully yielded a 10.2 kb viral genomic sequence. Subsequent in - depth analysis revealed high similarity to the YF vaccine strain 17D-213 and classified the sequence within the West Africa II genotype, clustering with the 17D vaccine strain.
Conclusions and Implications for Public Health Practice: This case represented YF AEFI. The implementation of tNGS technology enables more precise and expeditious pathogen sequencing, providing critical evidence for accurate disease diagnosis and informed public health interventions.
-
Yellow fever (YF) is a mosquito-borne disease preventable through a highly effective, safe, and affordable vaccine (1). The YF vaccine, a freeze-dried live-attenuated virus preparation, typically confers lifelong immunity after a single dose (1-2). Despite its established safety record spanning more than 80 years, mild adverse effects including fever, flu-like symptoms, muscle aches, and chills have been occasionally reported (2). Vaccine recipients may experience viremia between 2 and 6 days post-vaccination (3), with peak viremia typically occurring between days 4 and 6 following immunization (3).
While Reverse Transcription-Polymerase Chain Reaction (RT-PCR) assay remains widely used for YF virus nucleic acid detection, it lacks the capability to differentiate between wild-type and vaccine strains. Targeted Next-Generation Sequencing (tNGS), which combines targeted enrichment techniques with high-throughput sequencing, offers enhanced detection sensitivity compared to metagenomic NGS for multiple known pathogenic microorganisms (4–5). This method employs either multiplex PCR amplification or oligonucleotide probe hybridization to enrich target pathogen genes. The hybrid capture approach demonstrates superior sensitivity and specificity, making it particularly valuable for analyzing complex sequences across large-scale and multi-gene target regions. Consequently, tNGS has become instrumental in biological evolution studies, gene phenotype research, and investigations of genetic and rare diseases (6).
-
On October 13, 2024, a 46-year-old Peruvian man tested positive for YF during entry screening at Shenzhen customs. Guangdong CDC received notification from Shenzhen CDC on the evening of October 14, 2024, prompting an immediate epidemiological investigation.
The patient arrived at Hong Kong Airport via Amsterdam at 16:00 on October 13, 2024, proceeded directly to Guangzhou City, Guangdong Province by vehicle, and checked in at his hotel at 22:00. During his brief 30-minute stop at Shenzhen Bay Customs for entry procedures, he presented with a fever of 38.8℃ but no other symptoms. The patient had received YF vaccination on October 7, 2024, as documented in his immunization certificate, and had no travel history to Africa in the preceding 4 weeks. Blood samples analyzed by the Shenzhen Bay Customs Laboratory using RT-PCR assay yielded positive results for YF virus RNA in serum on October 14, 2024, while tests for coronavirus disease 2019 (COVID-19), influenza, Mpox, dengue, Zika, West Nile virus, and malaria were negative. The patient was immediately transferred via emergency ambulance to Guangzhou Eighth People’s Hospital, Guangzhou Medical University, for isolation and symptomatic treatment. Upon admission, his temperature had normalized to 36.4 ℃, and he remained asymptomatic.
On October 15, 2024, Guangdong CDC confirmed YF virus infection in the patient’s blood sample received from Guangzhou CDC, detecting viral RNA using a RT-PCR kit [BioGerm (Qingdao), China] with a Ct value of 33. By October 17, 2024, tNGS analysis successfully yielded a 10.2 kb viral genomic sequence. BLAST analysis revealed high similarity to the YF vaccine strain 17D-213 (GenBank Accession Number: U17067.1), with only 10 single nucleotide polymorphism (SNP) sites. Phylogenetic analysis classified the sequence within the West Africa II genotype, clustering with the 17D vaccine strain and showing 99.89%–99.79% homology with vaccine strains, while demonstrating only 85.4%–84.9% similarity to wild-type YF virus strains from South America (Figure 1). The National Institute for Viral Diseases Control and Prevention, China CDC, subsequently confirmed these viral RNA findings. Based on this evidence, an expert panel consultation concluded that this case represented YF Adverse Events following Immunization (AEFI), and the patient was discharged at 22:00 on October 17, 2024.
-
Upon receiving confirmation of Yellow fever virus positivity, the Guangdong Provincial, Guangzhou Municipal, and Haizhu District CDCs collaborated to implement comprehensive control measures. These included immediate patient isolation in a mosquito-proof facility, systematic health surveillance of close contacts, and sustained vector monitoring and control in all potentially affected areas.
Investigation and Results
Public Health Response
-
This study documents China’s first imported suspected Yellow fever case in 2024. Through comprehensive analysis of epidemiological data, vaccination history, and laboratory findings, we determined that the patient's febrile symptoms and viremia were attributable to Yellow fever vaccine-associated adverse events.
The Eliminate Yellow fever Epidemics strategy represents a global coalition of partners and affected countries established to address YF challenges. As Peru is designated as a YF-risk region, international travelers must present proof of YF vaccination (1). The patient developed a fever of 38.8 ℃ at Shenzhen Bay Customs on October 13, 2024 — six days post-vaccination — with YF viral RNA consistently detected in serum samples across multiple independent laboratories. Whole-genome sequencing conclusively demonstrated high sequence homology with the vaccine strain rather than wild-type strains circulating in South America, aligning with previously documented post-vaccination viremia patterns.
According to World Health Organization guidelines, a confirmed YF case in an unvaccinated population constitutes an outbreak. The 2016 importation of 11 YF cases to China, all confirmed as wild-type virus infections, prompted enhanced YF surveillance and early warning systems (7). In response to the customs alert, we immediately implemented comprehensive epidemiological investigations and rigorous laboratory testing, which confirmed the vaccine-associated nature of this case. To minimize future vaccine-associated adverse events, we recommend earlier administration of YF vaccination for international travelers.
-
Received ethics approval from the Ethical Committee of National Institute for Viral Disease Control and Prevention, Chinese Center for Disease Control and Prevention (No. IVDC2023-19).
| Citation: |



 Download:
Download:




