-
Diarrhea remains a significant global public health challenge, particularly among children under 10 years of age, where it ranks as the third leading cause of disability-adjusted life years (1). In China, rotavirus group A (RVA) has emerged as the predominant viral etiology of diarrhea, ranking first among children and second among adults in terms of frequency (2). Between 2005 and 2018, China experienced a fluctuating but generally increasing trend in rotavirus diarrhea incidence (3). In Shanghai specifically, rotavirus represents the third most prevalent pathogen in pediatric diarrheal cases and the second most common in adult cases, highlighting its substantial public health impact (4-5).
Given the absence of specific therapeutic interventions for rotavirus infection, vaccination stands as the most effective preventive strategy against rotavirus transmission (6). Prior to 2024, Shanghai offered two voluntary, self-funded oral rotavirus vaccines: the Lanzhou lamb rotavirus vaccine (LLR), licensed in 2001, and the oral human attenuated pentavalent rotavirus vaccine (RotaTeq), licensed in 2018.
Evidence indicates that LLR vaccination confers population-level health benefits through the prevention of rotavirus gastroenteritis (7). However, the impact of RotaTeq vaccination on rotavirus infection among both pediatric and adult populations in China remains to be fully elucidated. This study aims to investigate the temporal changes in rotavirus prevalence and characterize the epidemiological and genetic features among diarrheal outpatients from 2017 to 2023.
-
A comprehensive active prospective surveillance system for diarrheal diseases in both adult and pediatric populations has been operational in Shanghai since 2012. The current surveillance network encompasses 28 sentinel hospitals strategically distributed across all 16 districts of Shanghai, including 6 dedicated pediatric sentinel facilities. The network integrates primary, secondary, and tertiary healthcare facilities to ensure comprehensive coverage. This study builds upon this established surveillance infrastructure. Detailed protocols regarding sentinel hospital selection criteria, case definitions, specimen collection and storage procedures, RNA extraction methodologies, and laboratory testing protocols have been previously documented (4-5).
-
Comprehensive pathogenic screening was conducted on stool samples to detect five viral pathogens (rotavirus, norovirus, astrovirus, sapovirus, and adenovirus) and twelve bacterial agents (Vibrio cholerae, Shigella spp., Salmonella spp., V. parahaemolyticus, Campylobacter jejuni, Yersinia enterocolitica, Campylobacter coli, EPEC, ETEC, EHEC, EAggEC, EIEC).
-
RVA detection employed a dual-screening approach using real-time reverse transcription polymerase chain reaction (rRT-PCR) with commercial kits from Shanghai Zhijiang Biotechnology Co. Ltd, and Jiangsu Shuoshi Biotechnology Co., Ltd, following manufacturer-specified protocols. For specimens yielding positive results with both rRT-PCR kits, VP4 and VP7 genomic regions were amplified through nested PCR using established reagents and primers as documented in the literature (8). Genotype identification was subsequently performed through capillary gel electrophoresis based on product size analysis, following previously described methodologies (8).
-
Statistical analyses and data visualization were conducted using SAS software (version 9.4, SAS Institute Inc., Cary, USA) and R software (version 4.3.1, R Foundation for Statistical Computing, Vienna, Austria). For continuous variables, analyses employed either the Wilcoxon rank-sum test or the Kruskal-Wallis test based on data distribution characteristics. Categorical variables were evaluated using the Pearson chi-square test, continuous corrected chi-square test, or Fisher’s exact test as appropriate. Pathogen-specific positivity rates were calculated as the ratio of positive samples to total samples tested for each respective pathogen. Temporal trends in positivity rates were assessed using the Mann-Kendall trend test. All statistical analyses were conducted using two-sided tests with statistical significance defined at P<0.05.
-
Between January 1, 2017, and December 31, 2023, the study enrolled 10,749 outpatients presenting with diarrhea. Among 2,331 pediatric cases aged 0–14 years, 2,328 underwent RVA screening, yielding a positivity rate of 6.62%. The detection rates for other viral and bacterial pathogens were 20.96% and 21.73%, respectively. Age-stratified analysis revealed significantly higher RVA positivity rates in infants aged 0–1 year (7.9%) and children aged 2–5 years (7.08%) compared to those aged 6–14 years (3.59%) (P=0.045). Notable variations in RVA positivity were observed across residential status categories, with rates of 13.48% in migrant populations, 10.53% in temporary residents, and 6.18% in long-term residents (P=0.004). RVA-positive patients demonstrated significantly higher Vesikari scores compared to RVA-negative cases (P=0.023), with a greater proportion of moderate to severe cases in the positive group (P=0.001) (Table 1).
Characteristics All diarrhea patients Diarrhea patients† RVA positive RVA negative RVA positive P 2017–2018 2019 2020–2023 P Children No. 2,331 2,174 154 86 46 22 Gender 0.603 0.002 Male 1,293 (55.47) 1,202 (55.29) 89 (57.79) 39 (45.35) 34 (73.91) 16 (72.73) Female 1038 (44.54) 972 (44.71) 65 (42.21) 47 (54.65) 12 (26.09) 6 (27.27) Age group (years) 0.045 0.031 0–1 1,407 (60.36) 1,303 (59.94) 103 (66.88) 57 (66.28) 33 (71.74) 13 (59.09) 2–5 606 (26.00) 565 (25.99) 40 (25.97) 27 (31.40) 8 (17.39) 5 (22.73) 6–14 318 (13.64) 306 (14.08) 11 (7.14) 2 (2.33) 5 (10.87) 4 (18.18) Census register 0.004 0.059 Long-term residents 1,894 (81.25) 1,781 (81.92) 110 (71.43) 54 (62.79) 37 (80.43) 19 (86.36) Temporary residents 336 (14.41) 304 (13.98) 32 (20.78) 21 (24.42) 8 (17.39) 3 (13.64) Migrant population 101 (4.33) 89 (4.09) 12 (7.79) 11 (12.79) 1 (2.17) 0 (0.00) Vesikari score 5.00 (4.00, 6.00) 5.00 (4.00, 6.00) 6.00 (4.00, 7.00) 0.023 5.00 (4.00, 6.00) 6.00 (5.00, 7.00) 6.00 (5.00, 6.75) 0.004 Vesikari severity 0.001 0.240 Mild 2,240 (96.10) 2,098 (96.50) 139 (90.26) 81 (94.19) 39 (84.78) 19 (86.36) Moderate 71 (3.05) 59 (2.71) 12 (7.79) 4 (4.65) 6 (13.04) 2 (9.09) Severe 20 (0.86) 17 (0.78) 3 (1.95) 1 (1.16) 1 (2.17) 1 (4.55) Frequency of diarrhea (times/day) 4.00 (3.00, 5.00) 4.00 (3.00, 5.00) 4.00 (3.00, 5.00) 0.598 4.00 (3.00, 5.00) 4.00 (3.00, 6.00) 4.50 (3.00, 6.00) 0.203 Frequency of diarrhea (times/day) 0.766 0.074 3–4 1,240 (59.62) 1,154 (59.48) 85 (61.15) 53 (69.74) 23 (51.11) 9 (50.00) ≥5 840 (40.38) 786 (40.52) 54 (38.85) 23 (30.26) 22 (48.89) 9 (50.00) Fever <0.001 0.523 Yes 205 (8.79) 179 (8.23) 26 (16.88) 12 (13.95) 9 (19.57) 5 (22.73) Vomiting <0.001 0.538 Yes 177 (7.59) 148 (6.81) 28 (18.18) 13 (15.12) 10 (21.74) 5 (22.73) Adults No. 8,418 7,680 413 286 90 37 Gender 0.3335 0.543 Male 4,447 (52.83) 4,065 (52.93) 208 (50.36) 149 (52.10) 41 (45.56) 18 (48.65) Female 3,971 (47.17) 3,615 (47.07) 205 (49.64) 137 (47.90) 49 (54.44) 19 (51.35) Age group (years) 0.028 0.496 15–29 1,956 (23.32) 1,800 (23.52) 75 (18.29) 55 (19.43) 12 (13.33) 8 (21.62) 30–59 4,105 (48.94) 3,730 (48.73) 223 (54.39) 151 (53.36) 55 (61.11) 17 (45.95) ≥60 2,326 (27.73) 2,124 (27.75) 112 (27.32) 77 (27.21) 23 (25.56) 12 (32.43) Census register 0.157 0.631 Long-term residents 6,862 (81.52) 6,252 (81.41) 351 (84.99) 244 (85.31) 78 (86.67) 29 (78.38) Temporary residents 1,007 (11.96) 909 (11.84) 42 (10.17) 29 (10.14) 7 (7.78) 6 (16.22) Migrant population 549 (6.52) 519 (6.76) 20 (4.84) 13 (4.55) 5 (5.56) 2 (5.41) Frequency of diarrhea (times/day) 5.00 (4.00, 8.00) 5.00 (4.00, 8.00) 6.00 (4.00, 10.00) <0.001 6.00 (4.00, 10.00) 6.00 (4.00, 10.00) 8.00 (6.00, 10.00) 0.028 Frequency of diarrhea (times/day) <0.001 0.062 3–4 2,878 (35.78) 2,644 (36.06) 110 (27.23) 81 (29.03) 25 (28.41) 4 (10.81) ≥5 5,165 (64.22) 4,689 (63.94) 294 (72.77) 198 (70.97) 63 (71.59) 33 (89.19) Fever 0.262 0.863 Yes 1,184 (14.07) 1,084 (14.11) 67 (16.22) 48 (16.78) 14 (15.56) 5 (13.51) Vomiting <0.001 0.447 Yes 1,941 (23.06) 1,737 (22.62) 136 (32.93) 97 (33.92) 25 (27.78) 14 (37.84) Abbreviation: RVA=Rotavirus group A; IQR=interquartile range.
* Values are presented as N (%) or Median (IQR).
† RVA testing was not conducted for 325 adult and 3 pediatric diarrhea cases.Table 1. Demographic and clinical characteristics of pediatric and adult diarrhea outpatients, stratified by RVA test results and temporal periods*.
In the adult cohort (≥15 years, n=8,418), 8,093 specimens were tested for RVA, with a positivity rate of 5.10%, significantly lower than the pediatric cohort (P=0.005). Other viral and bacterial pathogens were detected at rates of 22.25% and 24.26%, respectively. Age-stratified analysis revealed significant variations in RVA positivity (P=0.028), with the highest rate observed in adults aged 30–59 years (5.98%) and the lowest in those aged 15–29 years (4.17%). Clinical presentation analysis showed that RVA-positive cases exhibited increased frequency of diarrhea and higher incidence of vomiting compared to RVA-negative cases (Table 1).
-
The overall RVA positivity rate across all age groups from 2017 to 2023 was 5.44%. A significant declining trend was observed, from 7.43% in 2017 and 7.77% in 2018 to 5.76% in 2019, followed by further reductions to 2.2%, 1.93%, 3.24%, and 1.19% from 2020 to 2023, respectively (P=0.024). The most pronounced decline occurred in children aged 2–5 years, where rates decreased significantly from 13.08% in 2017 to 1.72% in 2023 (P=0.024). Among infants aged 0–1 year, although rates decreased from 6.74% to 3.77%, this reduction was not statistically significant. In the 30–59 year age group, RVA positivity rates showed a significant reduction from 8.36% in 2017 to 1.06% in 2023 (P=0.004). Similarly, cases aged 60 years and over demonstrated a significant decline from 6.88% in 2017 to 0.48% in 2023 (P=0.011). Notably, the overall positivity rates for bacterial and other viral pathogens did not exhibit a decreasing trend during the study period. Despite temporary reductions during periods of non-pharmacological interventions, by 2023, these rates had returned to or slightly exceeded their 2017 levels (Figure 1).
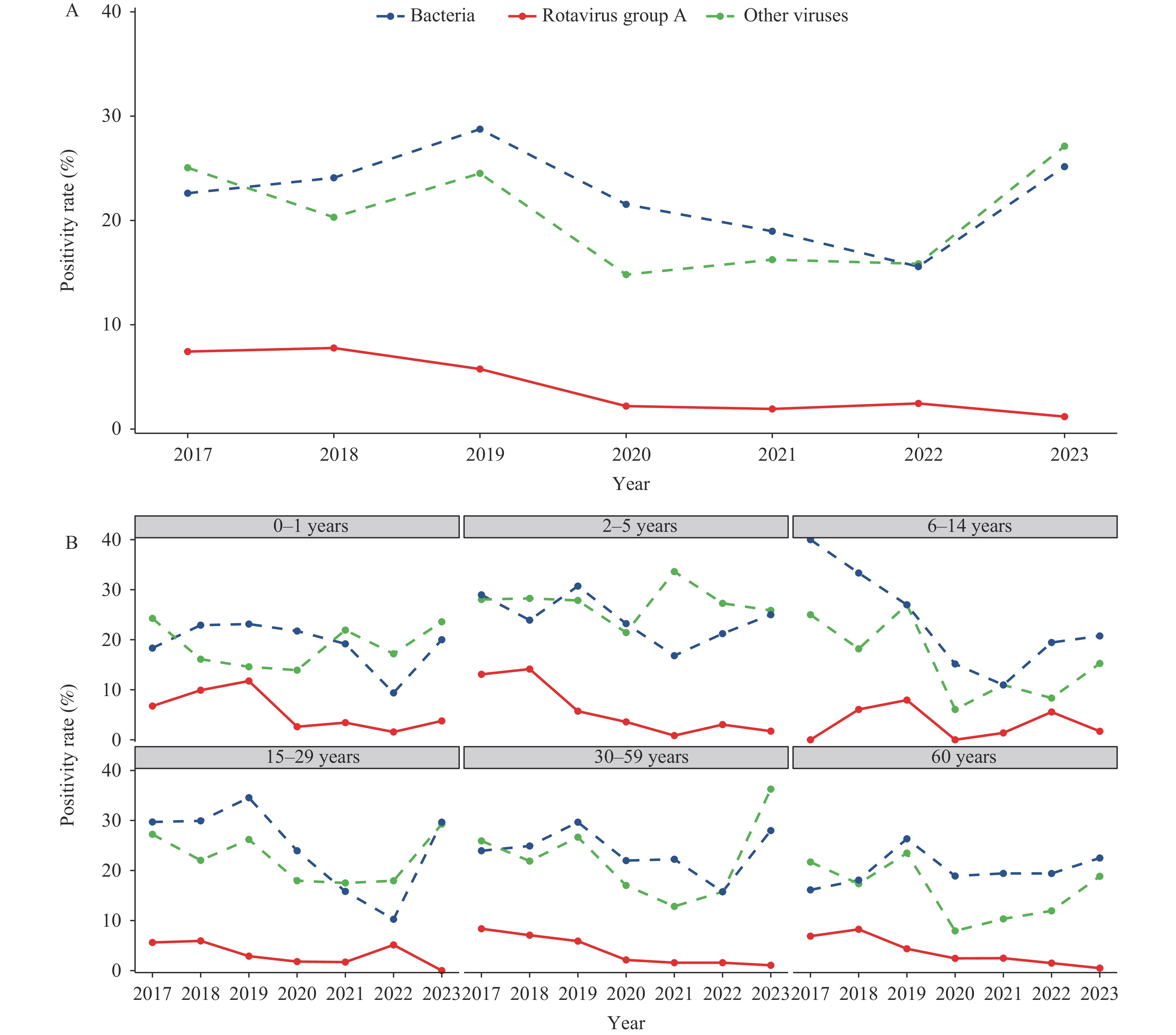 Figure 1.
Figure 1.Variation in positivity rates over different years, both overall and within various age groups. (A) variation in overall positivity rates over different years. (B) variation in positivity rates over different years within various age groups.
Note: The total positivity rates for bacteria were 22.61% in 2017, 24.09% in 2018, 28.75% in 2019, 21.54% in 2020, 18.96% in 2021, 15.57% in 2022, and 25.16% in 2023. The total positivity rates for other viruses were 25.06% in 2017, 20.31% in 2018, 24.51% in 2019, 14.81% in 2020, 16.24% in 2021, 15.85% in 2022, and 27.12% in 2023. -
Seasonal patterns of RVA in diarrhea cases were analyzed across three distinct time periods (2017–2018, 2019, and 2020–2023) for two age cohorts: individuals aged 5 and under, and those aged 15 and above.
Among children aged 5 and under, RVA positivity rates in 2017–2018 reached their peak during January and February, with rates of 30.43% to 31.34%. The 2019 period exhibited a sharp, concentrated peak of 41.38% in February, followed by a precipitous decline. In contrast, during 2020–2023, the maximum rate occurred in January at just 7.41%, representing only 23.64% and 17.91% of the peak rates observed in the two preceding periods, respectively (Figure 2).
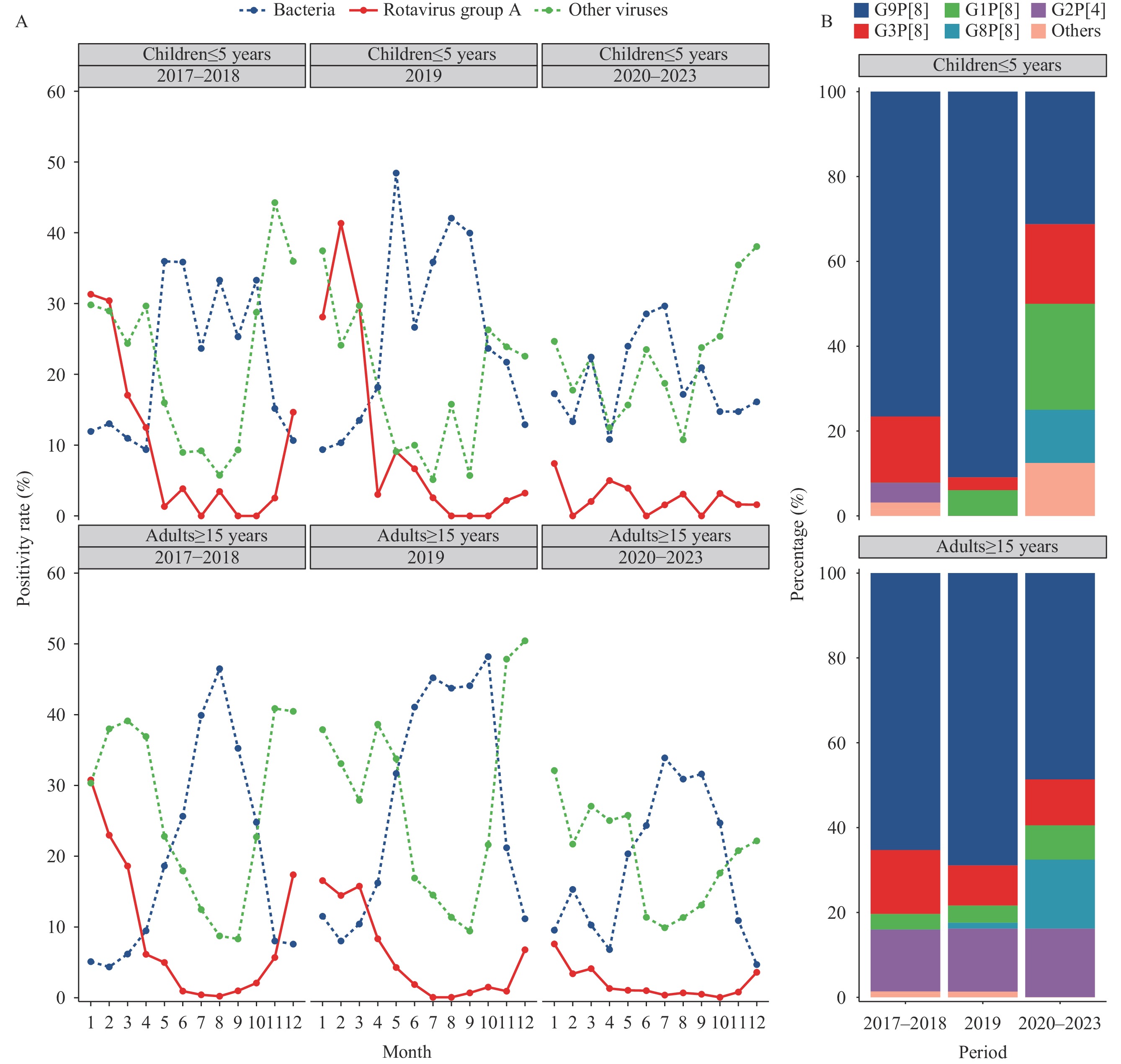 Figure 2.
Figure 2.Variation of positivity rate and genotype spectrum across different (A) months and (B) periods in adults and children over three time periods, 2017–2023.
For individuals aged 15 and older, the 2017–2018 period demonstrated a pronounced peak of 30.74% in January, followed by a swift decline. The 2019 period exhibited a broader seasonal pattern, with elevated rates persisting from January through March (ranging from 14.41% to 16.5%), characterized by lower peak intensity but extended duration compared to the previous period. During 2020–2023, the January peak diminished to 7.55%, representing only 24.56% and 45.76% of the peak rates observed in the two preceding periods, respectively (Figure 2).
-
Analysis of diarrhea cases in children aged 5 and younger revealed significant age distribution variations across the three time periods (P=0.031). The proportion of cases aged 0–1 years and 6–14 years shifted markedly in 2020–2023, reaching 59.09% and 18.18% respectively, compared to 66.28% and 2.33% in 2017–2018. Vesikari scores also showed significant differences across the three periods (P=0.004), with the lowest scores observed in 2017–2018. However, when categorized by severity using the Vesikari scoring system, these differences did not reach statistical significance (Table 1).
Among diarrhea cases aged 15 and older, the frequency of diarrheal episodes differed significantly across the three time periods (P=0.028), with the highest frequency documented during 2020–2023. No other clinical or demographic parameters showed statistically significant differences across these periods (Table 1).
-
Among 112 successfully genotyped RVA samples from children aged five and under, G9P[8] and G3P[8] emerged as the predominant strains, accounting for 75% and 12.5% of cases, respectively. The genotype composition underwent significant changes across the three time periods (P<0.001). From 2017–2018 to 2020–2023, the proportion of G9P[8] decreased markedly from 77.78% to 31.25%, while previously undetected strains G1P[8] and G8P[8] emerged in the population (Table 2, Figure 2).
RVA genotypes 2017–2018 n (%) 2019 n (%) 2020–2023 n (%) Total (2017–2023) n (%) Children ≤5 years G9P[8] 49 (77.78) 30 (90.91) 5 (31.25) 84 (75.00) G3P[8] 10 (15.87) 1 (3.03) 3 (18.75) 14 (12.50) G1P[8] 0 (0) 2 (6.06) 4 (25.00) 6 (5.36) G8P[8] 0 (0) 0 (0) 2 (12.50) 2 (1.79) G2P[4] 3 (4.76) 0 (0) 0 (0) 3 (2.68) G1P[8]+G3P[8] 0 (0) 0 (0) 1 (6.25) 1 (0.89) LLR vaccine strain 1 (1.59) 0 (0) 0 (0) 1 (0.89) RotaTeq vaccine strain 0 (0) 0 (0) 1 (6.25) 1 (0.89) Total 63 (100) 33 (100) 16 (100) 112 (100) Adults ≥15 years G9P[8] 143 (65.30) 51 (68.92) 18 (48.65) 212 (64.24) G3P[8] 33 (15.07) 7 (9.46) 4 (10.81) 44 (13.33) G1P[8] 8 (3.65) 3 (4.05) 3 (8.11) 14 (4.24) G8P[8] 0 (0) 1 (1.35) 6 (16.22) 7 (2.12) G2P[4] 32 (14.61) 11 (14.86) 6 (16.22) 49 (14.85) G9P[4] 2 (0.91) 0 (0) 0 (0) 2 (0.61) G2P[4]+G9P[8] 1 (0.46) 0 (0) 0 (0) 1 (0.30) G9P[8]+G2P[4] 0 (0) 1 (1.35) 0 (0) 1 (0.30) Total 219 (100) 74 (100) 37 (100) 330 (100) Table 2. Genotype constitution in children and adult RVA positive diarrhea outpatients over three time periods, 2017–2023.
Analysis of 330 successfully genotyped RVA samples from individuals aged 15 years and older revealed G9P[8], G2P[4], and G3P[8] as the dominant strains, representing 64.23%, 14.85%, and 13.33% of cases, respectively. The genotype distribution exhibited significant temporal variation across the three time periods (P<0.001). From 2017–2018 to 2020–2023, G9P[8] prevalence decreased from 65.3% to 48.65%, while G8P[8] emerged and increased to 16.22%. The proportion of G2P[4] remained relatively stable throughout this period (Table 2, Figure 2).
-
Clinical manifestations were significantly more severe in both adult and pediatric outpatients who tested positive for RVA compared to those who tested negative. The highest positivity rates were observed in children aged 5 years and younger, with particular vulnerability noted among infants under 1 year of age. These findings underscore the critical importance of implementing effective rotavirus prevention strategies, especially for these high-risk age groups.
Since 2018–2019, RVA positivity rates have shown differential declines across age groups, with the most pronounced reduction observed in children aged 2–5 years. By 2023, the RVA positivity rate in this group had decreased dramatically to 1.72% from 13.08% in 2017, representing an 11.36 percentage point reduction. Similar declining trends were documented in adult populations. These findings align with data reported from multiple Beijing hospitals (9). In Shanghai, the introduction of RotaTeq in 2018 coincided with a sharp decline in LLR coverage among infants born in 2020, dropping from 66% to 11.8%, while RotaTeq coverage rapidly exceeded 40% (10). Comparable trends have been observed internationally; in Japan, the introduction of Rotarix and RotaTeq as optional immunizations led to a reduction in RVA positivity rates from 20% to 4% within two years (11). This pattern is consistent with global data showing decreased rotavirus prevalence among acute gastroenteritis cases following widespread vaccine implementation (12).
The stability of bacterial and other viral pathogen positivity rates from 2017 to 2023, returning to baseline levels by 2023, suggests that the decline in RVA positivity rates was not attributable to temporal variations in testing methodology or non-pharmacological interventions implemented during 2020–2022. Notably, transient populations exhibited approximately double the positivity rates of permanent residents. The limited effectiveness of improved hygiene measures in controlling rotavirus transmission (6), coupled with vaccination coverage among transient populations being less than half that of permanent residents (10), highlights the crucial relationship between vaccination coverage and infection rates. These findings strongly suggest that the introduction of RotaTeq as a voluntary vaccine has been instrumental in reducing RVA infections among children since 2018–2019.
A notable decline in RVA positivity rates among adult diarrhea outpatients suggests that pediatric rotavirus vaccination may confer indirect population-wide protection against infection. This observation aligns with studies from America demonstrating that childhood rotavirus immunization provides indirect protective effects for unvaccinated cohorts, including both pediatric and adult populations (13-14).
From 2017 to 2023, the age distribution of rotavirus diarrhea cases exhibited a significant shift toward older pediatric groups, a pattern consistent with observations in other countries (12). Specifically, the period from 2017–2018 to 2020–2023 saw a decrease in both the absolute number and proportion of cases among infants aged 0–1 years. Conversely, while the absolute number of cases in children aged 6–14 years remained relatively stable, their proportional representation increased. The typical seasonal epidemic pattern of rotavirus, characterized by January peaks, has shown diminishing peak intensity since 2018.
The surveillance period from 2017 to 2023 revealed a marked decline in G9P[8] strain predominance, concurrent with the emergence of G8P[8]. The most pronounced reduction in G9P[8] prevalence occurred among children aged 5 years and younger, though it maintained its position as the dominant strain from 2020 to 2023. Studies from northern and central China have reported similar trends, noting a transition from G9P[8] to G8P[8] as the predominant strain during 2021–2022 (9,15). Genetic analyses identified G9P[8], G1P[8], G3P[8], and G8P[8] as the prevalent RVA strains in Shanghai, indicating substantial genetic diversity. The emergence of G8P[8] strains may be associated with rotavirus vaccine implementation (11,15). Continued surveillance is essential to monitor the potential persistence of this G8P[8] strain shift in Shanghai.
This study presents several limitations. First, its focus on outpatient cases reflects the healthcare practices in Shanghai, where prompt treatment of pediatric diarrhea typically prevents hospitalization, resulting in limited inpatient data. Second, the use of positivity rates without community disease burden assessment provides only a measure of infection prevalence intensity. Third, non-pharmacological interventions, including reduced population mobility and enhanced infectious disease prevention awareness, led to decreased medical consultations for diarrhea and reduced surveillance cases. Consequently, the lower number of diarrhea cases and RVA-positive samples during this period constrains the ability to conduct comprehensive annual analyses of seasonal patterns and genotype distribution.
Given the recent licensing and completion of phase III clinical trials for multiple domestically produced vaccines, maintaining vigilant surveillance of rotavirus diarrhea epidemiological trends and genotypes, coupled with real-world vaccine effectiveness evaluations, is crucial. These efforts will generate essential scientific evidence to optimize rotavirus immunization strategies.
-
The essential contributions of public health workers from the municipal and district Centers for Disease Control and Prevention and healthcare workers from surveillance hospitals.
-
Received approval from the Institutional Ethical Review Committee of the Shanghai Municipal Centers for Disease Control and Prevention (2022-38).
HTML
Shanghai Diarrhea Comprehensive Surveillance
Laboratory Tests
RVA Genotyping
Statistical Analysis
Characteristics of Diarrhea Outpatients
Changes in RVA Prevalence
Seasonal Pattern of RVA Circulation
Characteristics of RVA Positive Diarrhea Cases
RVA genotype diversity
| Citation: |



 Download:
Download:




