-
Schizophrenia represents a severe mental disorder with substantial global disease burden. According to the Global Burden of Diseases, Injuries, and Risk Factors Study (GBD) 2019, more than 20 million people worldwide live with schizophrenia, with the condition accounting for 12.2% of disability-adjusted life years across all mental disorders (1). The disorder exhibits a strong association with suicide (2), with lifetime prevalence rates of 26.8% for suicide attempts and approximately 5% for suicide deaths among individuals with schizophrenia (3). Identifying patients at risk of suicide to enable targeted interventions remains a significant clinical challenge. While extensive research has examined suicide risk factors in schizophrenia patients, the relationships between psychiatric symptoms, sleep disturbances, and suicide risk remain poorly understood in hospitalized populations. Notably, hospitalized patients face particularly high suicide risk, with approximately one-third of suicide behaviors in schizophrenia patients occurring during hospitalization or within one week of discharge, and elevated risk persisting throughout the first post-discharge year (2). This underscores the critical importance of suicide risk assessment at admission. This study investigated suicide risk patterns in hospitalized schizophrenia patients and identified associated factors across comprehensive psychopathological dimensions, including multiple psychiatric symptom domains and sleep disturbances. The findings indicate higher suicide risk prevalence among schizophrenia inpatients experiencing psychiatric symptoms and sleep disturbances. These results suggest that systematic evaluation and intervention targeting these symptoms may help guide clinical practice and improve suicide risk management in this population.
This cross-sectional study employed convenience sampling to recruit schizophrenia inpatients from nine hospitals across Beijing Municipality and Henan, Hebei, and Shandong provinces in China from August 2019 to July 2022. The Ethics Committee of Peking University Sixth Hospital approved the protocol (No. 2019-18), and all participants provided written informed consent. Eligible participants met the International Classification of Diseases (tenth edition) diagnostic criteria for schizophrenia, confirmed by the Mini-International Neuropsychiatric Interview (M.I.N.I.). Inclusion criteria required participants to be 18 years or older, have at least primary education, and score 20 or higher on the Mini-Mental State Examination (MMSE). Exclusion criteria encompassed severe cardiac, hepatic, nephritic, or respiratory dysfunction and other serious diseases. This study collected general demographic data and clinical characteristics. Psychiatric symptoms were evaluated using the Brief Psychiatric Rating Scale (BPRS), an 18-item scale ranging from 18 to 126 points, with higher scores indicating greater symptom severity. The BPRS comprises 5 factors: withdrawal-retardation, thinking disorder, anxious-depression, hostile-suspiciousness, and activation. Depressive and anxiety symptoms were assessed using the Patient Health Questionnaire-9 (PHQ-9) and Generalized Anxiety Disorder-7 (GAD-7) self-rated scales, respectively. Sleep disturbances were evaluated using the Pittsburgh Sleep Quality Index (PSQI) and Epworth Sleepiness Scale (ESS), while cognitive performance was assessed using the Montreal Cognitive Assessment (MoCA). Suicide risk was categorized as none, mild, or moderate to severe based on scores from the M.I.N.I. (version 6.0) suicide module, administered by uniformly trained psychiatrists. The primary outcome was current suicide risk, defined as mild, moderate, or severe risk. Secondary outcomes included current suicidal ideation, suicide plan, and suicide attempt in the past month, as determined by specific questions in the M.I.N.I. suicide module. Of 714 invited inpatients, 672 participants (94.1% response rate) provided valid data and were included in the final analysis.
Measurement data of normal distribution were expressed as x±s, and an independent sample t-test was used to compare the two groups. Non-normally distributed data were represented by M (Q1, Q3), and the Mann-Whitney U test was used to compare groups. Count data were expressed as n (%), and the χ2 test was used for comparisons. A multivariable logistic regression was applied to obtain independent associated factors of suicide risk. All statistical methods used a two-tailed test, and differences where P<0.05 were considered statistically significant. All statistical analyses were performed using SPSS software (version 25.0. IBM Corp., Armonk, NY, USA).
Among the hospitalized patients with schizophrenia, the median age was 38 years, with females comprising 33.0% (222/672) of the cohort. All patients were receiving antipsychotic medication. The prevalence of PHQ-9-defined depressive symptoms and GAD-7-defined anxiety symptoms was 12.4% (83/672) and 7.9% (53/672), respectively. Sleep disturbances were common, with 56.8% (382/672) of patients experiencing poor sleep quality (PSQI score >5) and 19.2% (129/672) reporting daytime sleepiness (ESS score >10). Mild cognitive impairment (MCI) was present in 77.8% (523/672) of patients, as defined by a MoCA score below 26.
The overall prevalence of suicide risk in hospitalized patients with schizophrenia was 22.3% [95% confidence interval (CI): 19.3%, 25.6%], with 14.6% (98/672) classified as mild risk and 7.8% (52/672) as moderate to severe risk. Current suicidal ideation was present in 10.9% (73/672) of patients, while 2.5% (17/672) reported suicide plans and 3.3% (22/672) had attempted suicide.
Analysis of demographic and clinical characteristics revealed that patients with suicide risk were significantly younger than those without risk (median age 35 years vs. 39 years, P=0.004). Age stratification showed a higher proportion of younger patients (18–35 years) in the suicide risk group (53.3% vs. 41.4%, P=0.010). Additionally, patients with suicide risk had a higher rate of previous modified electroconvulsive therapy (35.3% vs. 23.8%, P=0.005). No significant differences were observed between groups regarding sex distribution, education level, body mass index (BMI), or marital status (Table 1).
Variable Total (N=672) Schizophrenia with suicide risk (N=150) Schizophrenia without suicide risk (N=522) χ2/Z-value P Demographic characteristics Sex [n (%)] 0.253 0.615 Male 450 (67.0) 103 (68.7) 347 (66.5) Female 222 (33.0) 47 (31.3) 175 (33.5) Age [years, M (Q1, Q3)] 38.00 (30.00, 49.00) 35.00 (27.00, 45.00) 39.00 (30.00, 49.00) −2.899 0.004 Age group (years) 9.184 0.010 18–35 [n (%)] 296 (44.0) 80 (53.3) 216 (41.4) 36–60 [n (%)] 340 (50.6) 67 (44.7) 273 (52.3) >60 [n (%)] 36 (5.4) 3 (2.0) 33 (6.3) Marital status [n (%)] 1.112 0.292 Married 192 (28.6) 48 (32.0) 144 (27.6) Not married 480 (71.4) 102 (68.0) 378 (72.4) Education [n (%)] 0.934 0.334 College or above 121 (18.0) 23 (15.3) 98 (18.8) Lower than college 551 (82.0) 127 (84.7) 424 (81.2) BMI [kg/m2, M (Q1, Q3)] 24.77 (22.08, 27.69) 24.60 (21.88, 27.69) 24.82 (22.18, 27.70) −0.229 0.819 Clinical characteristics Disease duration [years, M (Q1, Q3)] 11.00 (5.00, 20.00) 10.00 (5.00, 16.00) 11.00 (5.00, 20.00) −1.698 0.089 Family history of mental disorders [n (%)] 0.489 0.485 Yes 97 (14.4) 19 (12.7) 78 (14.9) No 575 (85.6) 131 (87.3) 444 (85.1) History of drug allergy [n (%)] 0.195 0.659 Yes 23 (3.4) 6 (4.0) 17 (3.3) No 649 (96.6) 144 (96.0) 505 (96.7) History of alcohol consumption [n (%)] 2.541 0.111 Yes 40 (6.0) 13 (8.7) 27 (5.2) No 632 (94.0) 137 (91.3) 495 (94.8) History of smoking [n (%)] 2.350 0.125 Yes 161 (24.0) 43 (28.7) 118 (22.6) No 511 (76.0) 107 (71.3) 404 (77.4) MECT history [n (%)] 8.051 0.005 Yes 177 (26.3) 53 (35.3) 124 (23.8) No 495 (73.7) 97 (64.7) 398 (76.2) Current medication Antipsychotics [n (%)] 672 (100) 150 (100) 522 (100) NA NA Antidepressants [n (%)] 54 (8.0) 15 (10.0) 39 (7.5) 1.008 0.315 Sedative-hypnotics [n (%)] 195 (29.0) 42 (28.0) 153 (29.3) 0.097 0.755 Mood stabilizers [n (%)] 109 (16.2) 22 (14.7) 87 (16.7) 0.343 0.558 Sleep parameters PSQI [M (Q1, Q3)] Subjective sleep quality 1.00 (0, 1.00) 1.00 (1.00, 2.00) 1.00 (0, 1.00) −5.329 <0.001 Sleep latency 1.00 (1.00, 2.00) 1.00 (1.00, 2.00) 1.00 (0, 2.00) −2.071 0.038 Sleep duration 0 (0, 1.00) 0 (0, 1.00) 0 (0, 1.00) −3.224 0.001 Habitual sleep efficiency 0 (0, 1.00) 0 (0, 2.00) 0 (0, 1.00) −2.395 0.017 Sleep disturbances 1.00 (1.00, 1.00) 1.00 (1.00, 1.00) 1.00 (0, 1.00) −4.513 <0.001 Use of sleeping medication 1.00 (0, 3.00) 2.00 (0, 3.00) 1.00 (0, 3.00) −2.618 0.009 Daytime dysfunction 1.00 (0, 2.00) 2.00 (1.00, 3.00) 1.00 (0, 2.00) −6.243 <0.001 Total score 6.00 (3.00, 10.00) 8.50 (5.00, 11.00) 6.00 (3.00, 9.00) −5.656 <0.001 Poor sleep quality [n (%)] 21.400 <0.001 Yes 382 (56.8) 110 (73.3) 272 (52.1) No 290 (43.2) 40 (26.7) 250 (47.9) ESS total score [M (Q1, Q3)] 5.00 (1.00, 9.00) 6.00 (2.00, 11.00) 5.00 (0, 9.00) −3.094 0.002 Daytime sleepiness [n (%)] 4.689 0.030 Yes 129 (19.2) 38 (25.3) 91 (17.4) No 543 (80.8) 112 (74.7) 431 (82.6) Psychiatric symptoms MoCA total score [M (Q1, Q3)] 22.00 (18.00, 25.00) 22.00 (18.00, 25.00) 22.00 (18.00, 25.00) −0.847 0.397 Mild cognitive impairment [n (%)] 1.375 0.241 Yes 523 (77.8) 122 (81.3) 401 (76.8) No 149 (22.2) 28 (18.7) 121 (23.2) PHQ-9 total score [M (Q1, Q3)] 3.00 (0, 7.00) 8.00 (4.00, 12.00) 2.00 (0, 4.00) −11.399 <0.001 Depressive symptom [n (%)] 73.619 <0.001 Yes 83 (12.4) 49 (32.7) 34 (6.5) No 589 (87.6) 101 (67.3) 488 (93.5) GAD-7 total score [M (Q1, Q3)] 1.00 (0, 4.75) 5.50 (1.00, 10.00) 0.50 (0, 3.00) −9.951 <0.001 Anxiety symptom [n (%)] 87.206 <0.001 Yes 53 (7.9) 39 (26.0) 14 (2.7) No 619 (92.1) 111 (74.0) 508 (97.3) BPRS [M (Q1, Q3)] Withdrawal-retardation 2.00 (1.50, 2.50) 2.00 (1.50, 2.50) 2.25 (1.50, 2.50) −0.457 0.648 Thinking disorder 1.50 (1.00, 2.25) 1.50 (1.00, 2.25) 1.75 (1.00, 2.50) −2.093 0.036 Anxious-depression 1.75 (1.00, 2.50) 2.50 (2.00, 3.00) 1.50 (1.00, 2.06) −9.502 <0.001 Hostile-suspiciousness 2.00 (1.00, 2.67) 2.33 (1.00, 2.67) 2.00 (1.00, 2.67) −1.010 0.313 Activation 1.00 (1.00, 1.67) 1.67 (1.00, 1.67) 1.00 (1.00, 1.67) −5.155 <0.001 Total score 33.00 (27.00, 38.00) 35.00 (30.00, 40.00) 32.00 (27.00, 38.00) −3.824 <0.001 Note: Poor sleep quality is defined as PSQI >5. Daytime sleepiness is defined as ESS >10. MCI is defined as MoCA <26. Depressive symptom is defined as PHQ-9 ≥10. Anxiety symptom is defined as GAD-7 ≥10. Continuous variables not conforming to the normal distribution were expressed as M (Q1, Q3); categorical variables were expressed as n (%).
Abbreviation: M=median; Q1=lower quartile; Q3=upper quartile; BMI=body mass index; MECT=modified electroconvulsive therapy; PSQI=Pittsburgh Sleep Quality Index; ESS=Epworth Sleepiness Scale; MoCA=Montreal Cognitive Assessment; BPRS=Brief Psychiatric Rating Scale; PHQ-9=Patient Health Questionnaire-9; GAD-7=Generalized Anxiety Disorder-7.Table 1. Demographic characteristics, clinical characteristics, sleep parameters and psychiatric symptoms of schizophrenia inpatients with and without suicide risk.
Regarding psychiatric symptoms and sleep disturbances, patients with suicide risk exhibited significantly higher rates of poor sleep quality (73.3% vs. 52.1%, P<0.001), daytime sleepiness (25.3% vs. 17.4%, P=0.030), depressive symptoms (32.7% vs. 6.5%, P<0.001), and anxiety symptoms (26.0% vs. 2.7%, P<0.001) compared to those without suicide risk. Total BPRS scores, factor scores (anxious-depression and activation), PHQ-9, GAD-7, PSQI, and ESS scores were also significantly elevated (all P<0.01), while the thinking disorder factor score was lower (P=0.036) (Table 1). Among patients with varying levels of suicide risk, BPRS total scores, PHQ-9, GAD-7, and PSQI scores showed positive correlations (Figure 1A), and the prevalence of depressive symptoms, anxiety symptoms, and poor sleep quality increased proportionally with suicide risk level (Figure 1B). Neither the level nor the prevalence of daytime sleepiness showed significant increases with elevated suicide risk.
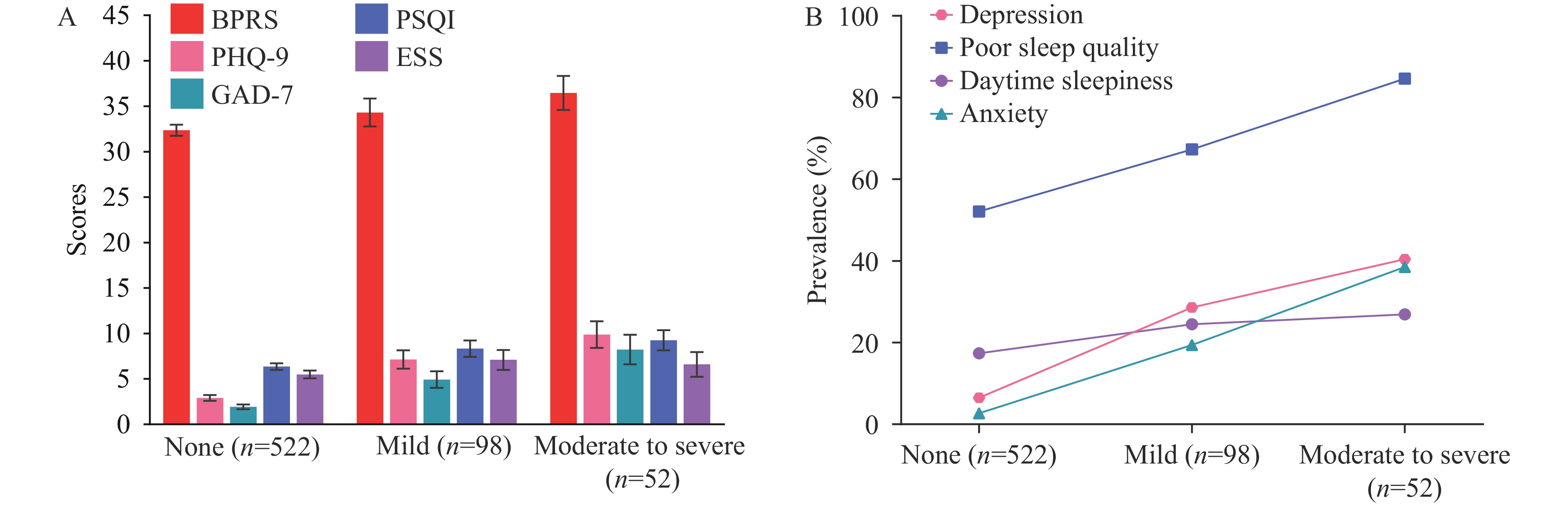 Figure 1.
Figure 1.Psychiatric symptoms and sleep disturbances in schizophrenia inpatients with different suicide risk levels. (A) Scores; (B) Prevalence.
Note: Error bars indicate 95% CIs. Suicide risk was categorized as none, mild, or moderate to severe according to the Mini-International Neuropsychiatric Interview suicide module assessment by trained psychiatrists. Anxiety is defined as GAD-7≥10. Depression is defined as PHQ-9≥10. Poor sleep quality is defined as PSQI>5. Daytime sleepiness is defined as ESS>10.
Abbreviation: BPRS=Brief Psychiatric Rating Scale; PHQ-9=Patient Health Questionnaire-9; GAD-7=Generalized Anxiety Disorder-7; PSQI=Pittsburgh Sleep Quality Index; ESS=Epworth Sleepiness Scale; CI=confidence interval.
In the multivariable logistic regression analysis, multiple potential contributors to suicide risk were considered including: sex, age group, marital status, education, BMI, disease duration, family history of mental disorders, history of drug allergy, histories of smoking and alcohol consumption, modified electroconvulsive therapy history, MCI, anxiety symptoms, depressive symptoms, poor sleep quality, daytime sleepiness, and BPRS factor scores (withdrawal-retardation, thinking disorder, hostile-suspiciousness, and activation). The anxious-depression factor of BPRS was excluded due to the presence of PHQ-9-defined depressive symptoms and GAD-7-defined anxiety symptoms. The analysis revealed that suicide risk in schizophrenia was independently associated with poor sleep quality [adjusted odds ratio (aOR)=2.09, 95% CI: 1.31, 3.36, P=0.002], depressive symptoms (aOR=2.24, 95% CI: 1.19, 4.23, P=0.013), anxiety symptoms (aOR=6.91, 95% CI: 3.10, 15.41, P<0.001), thinking disorder (aOR=0.55, 95% CI: 0.39, 0.80, P=0.002), and activation (aOR=1.85, 95% CI: 1.13, 3.04, P=0.014) (Table 2).
Variable Unadjusted OR (95% CI) P Adjusted OR (95% CI)* P Sex Male 1 – 1 – Female 0.91 (0.61, 1.34) 0.615 0.90 (0.54, 1.51) 0.680 Age group (years) 18–35 1 – 1 – 36–60 0.66 (0.46, 0.96) 0.029 0.80 (0.48, 1.33) 0.386 >60 0.25 (0.07, 0.82) 0.023 0.36 (0.09, 1.55) 0.171 Marital status Not married 1 – 1 – Married 1.24 (0.83, 1.83) 0.292 1.44 (0.89, 2.35) 0.141 Education Lower than college 1 – 1 – College or above 0.78 (0.48, 1.29) 0.335 1.02 (0.57, 1.80) 0.954 BMI (kg/m2) 1.00 (0.96, 1.04) 0.922 0.99 (0.94, 1.03) 0.518 Disease duration (years) 0.98 (0.96, 1.00) 0.030 1.00 (0.97, 1.02) 0.725 Family history of mental disorders No 1 – 1 – Yes 0.83 (0.48, 1.41) 0.485 1.05 (0.56, 1.98) 0.873 History of drug allergy No 1 – 1 – Yes 1.24 (0.48, 3.20) 0.660 0.63 (0.18, 2.22) 0.472 History of alcohol consumption No 1 – 1 – Yes 1.74 (0.87, 3.46) 0.115 1.18 (0.49, 2.89) 0.712 History of smoking No 1 – 1 – Yes 1.38 (0.91, 2.07) 0.126 1.22 (0.71, 2.10) 0.481 MECT history No 1 – 1 – Yes 1.75 (1.19, 2.59) 0.005 1.36 (0.84, 2.20) 0.209 Poor sleep quality No 1 – 1 – Yes 2.53 (1.69, 3.77) <0.001 2.09 (1.31, 3.36) 0.002 Daytime sleepiness No 1 – 1 – Yes 1.61 (1.04, 2.48) 0.031 1.02 (0.60, 1.72) 0.950 Mild cognitive impairment No 1 – 1 – Yes 1.32 (0.83, 2.08) 0.242 1.23 (0.72, 2.10) 0.454 Depressive symptom No 1 – 1 – Yes 6.96 (4.28, 11.33) <0.001 2.24 (1.19, 4.23) 0.013 Anxiety symptom No 1 – 1 – Yes 12.75 (6.69, 24.28) <0.001 6.91 (3.10, 15.41) <0.001 Withdrawal-retardation 0.94 (0.72, 1.22) 0.637 0.73 (0.52, 1.03) 0.070 Thinking disorder 0.73 (0.56, 0.94) 0.013 0.55 (0.39, 0.80) 0.002 Hostile-suspiciousness 1.06 (0.88, 1.29) 0.524 1.09 (0.82, 1.45) 0.537 Activation 2.46 (1.67, 3.63) <0.001 1.85 (1.13, 3.04) 0.014 Abbreviation: OR=odds ratio; CI=confidence interval; BMI=body mass index; MECT=modified electroconvulsive therapy.
* OR was adjusted for the variables listed above in a multivariable logistic regression model.Table 2. Unadjusted and adjusted ORs of associated factors of suicide risk in schizophrenia inpatients.
-
These findings demonstrate a high prevalence of suicide risk among hospitalized patients with schizophrenia, with significant associations between suicide risk and both psychiatric symptoms and sleep disturbances. The results indicate that schizophrenia inpatients with suicide risk are characterized by younger age, lower levels of thinking disorder, and higher levels of activation, coupled with anxiety symptoms, depressive symptoms, and poor sleep quality compared to those without suicide risk. These findings suggest that systematic evaluation and targeted intervention for these symptoms may help reduce suicide risk in clinical settings and improve both clinical practice and health management strategies.
Previous studies of schizophrenia patients in China have reported suicidal ideation prevalence rates ranging from 7.4% to 57.6% (4). Meta-analytic evidence indicates lifetime and point prevalence rates of suicidal ideation among schizophrenia patients of 34.5% and 29.9%, respectively, while lifetime and point prevalence rates of suicide plans were 44.3% and 6.4%–13%, respectively (5). The 1-month prevalence of suicide attempts in patients with schizophrenia was reported at 2.7% across studies (6). In this study, the prevalence of suicide risk (22.3%, 95% CI: 19.3%, 25.6%) was lower than anticipated, potentially due to the sample characteristics. Since this cohort consisted of hospitalized patients with extended disease duration, their psychiatric symptoms and sleep disorders were relatively mild post-treatment compared to those in acute episodes.
The relationship between psychiatric symptoms and suicide risk in schizophrenia patients has been extensively investigated. While some studies suggest that increased positive symptoms, such as hallucinations and delusions, are associated with suicide (7-8), evidence indicates that command auditory hallucinations specifically, rather than auditory hallucinations in general, correlate with suicidal behavior (8). The nature of psychotic symptoms is highly complex and variable, with marked individual differences. The elevated suicide risk may stem not from the psychotic symptoms themselves but from the accompanying distress, depression, and hopelessness (9). Suicide rates were notably higher among schizophrenia patients experiencing depression and anxiety, particularly depressed mood and hopelessness (2). This study reveals that suicide risk in schizophrenia inpatients is associated with multiple psychiatric symptoms: anxiety, depression, thinking disorder, and activation. These results underscore the critical importance of comprehensive assessment of both affective and positive symptoms in schizophrenia patients for timely identification of suicide risk.
Sleep disturbances are highly prevalent in schizophrenia, affecting up to 80% of patients, with manifestations including difficulties in initiating or maintaining sleep and excessive daytime sleepiness (10). Among these disturbances, insomnia represents the predominant sleep disorder in schizophrenia patients, occurring at significantly higher rates compared to the general population (11). This study demonstrates that both the prevalence and severity of poor sleep quality increased proportionally with suicide risk level, providing further evidence for the robust association between sleep disturbances and suicide risk in schizophrenia.
This investigation has three primary limitations. First, the cross-sectional study design only allows for the identification of correlative factors associated with suicide risk and cannot establish causality; additionally, recall bias remains an inherent challenge. Second, while the M.I.N.I. effectively assesses current suicide risk, it cannot predict progression to death by suicide. Third, the relatively small sample size constrains the study’s statistical power and generalizability, necessitating larger-scale investigations to validate these findings.
In conclusion, this study emphasizes the critical importance of suicide risk assessment in schizophrenia inpatients. Comprehensive monitoring of psychiatric symptoms and sleep disturbances can facilitate early identification of patients at elevated suicide risk, and timely intervention targeting these symptoms may help reduce suicide risk. When clinicians identify these symptoms in hospitalized schizophrenia patients, they should alert family members and community healthcare providers to maintain vigilant suicide prevention measures following discharge.
-
The project teams from Peking University Sixth Hospital, Beijing Changping District Hospital of Integrated Traditional Chinese and Western Medicine, Beijing Tongzhou Psychiatric Hospital, Beijing Chaoyang Third Hospital, Beijing Xicheng District Ping’an Hospital, Zhumadian Second People’s Hospital, Liaocheng Fourth People’s Hospital, Fifth Hospital of Qinhuangdao, and Kailuan Mental Health Centre, and all study participants. Yanping Bao for providing valuable guidance on statistical analysis.
HTML
| Citation: |



 Download:
Download:




