-
Scrub typhus, commonly known as Tsutsugamushi disease, is a natural-focal infectious disease caused by Orientia tsutsugamushi. The disease primarily circulates between rodents as reservoir hosts and chigger mite larvae as vectors, and remains the most underdiagnosed infectious disease globally. Clinical manifestations typically include acute onset, characteristic eschar or ulcers at the bite site, lymphadenopathy, and maculopapular rash (1). The disease is endemic throughout the Asia-Pacific region (including China, the Republic of Korea, Japan, and India), extends into northern Australia, and has emerged in Africa, southern Chile, and the Middle East (2-3). Here, this study reports the first documented case (2023) of Orientia tsutsugamushi infection identified in Ningxia Hui Autonomous Region, China.
A 58-year-old female farmer, who maintained livestock including cattle, sheep, and pigs near her residence along with domestic pets, presented with scrub typhus symptoms. Her home was situated in a hilly forested area surrounded by farmland with substantial rodent activity. The patient reported an insect bite on her right ankle on July 10, 2023, which developed into an itchy white rash that subsequently formed a scab after scratching. Three days post-bite, she experienced an acute onset of high fever (39.7 ℃) with accompanying headache, nausea, and localized inflammation at the bite site characterized by redness, swelling, itching, and pain. Though antipyretic medication reduced her temperature to 37℃, other symptoms persisted. Upon hospital admission on July 21, clinical examination revealed acute facial features, bilateral inguinal lymphadenopathy (right: 2.1 cm × 1.1 cm; left: 2.7 cm × 1 cm), and disseminated papular rash. The bite site displayed a small (0.15 cm), painful, black eschar on the medial aspect of the right ankle joint, surrounded by marked swelling (Figure 1). While Widal reaction and brucellosis tests were negative, laboratory findings showed decreased eosinophil (EO#), normal white blood cell (WBC) counts, and elevated hematocrit (HCT). Blood biochemistry revealed elevated C-reactive protein, increased myocardial enzymes, and elevated aspartate aminotransferase (AST) and alanine aminotransferase (ALT), indicating liver dysfunction (Table 1). Computed tomography confirmed bilateral inguinal lymphadenopathy (Table 1 and Figures 2A–B), supporting the scrub typhus diagnosis.
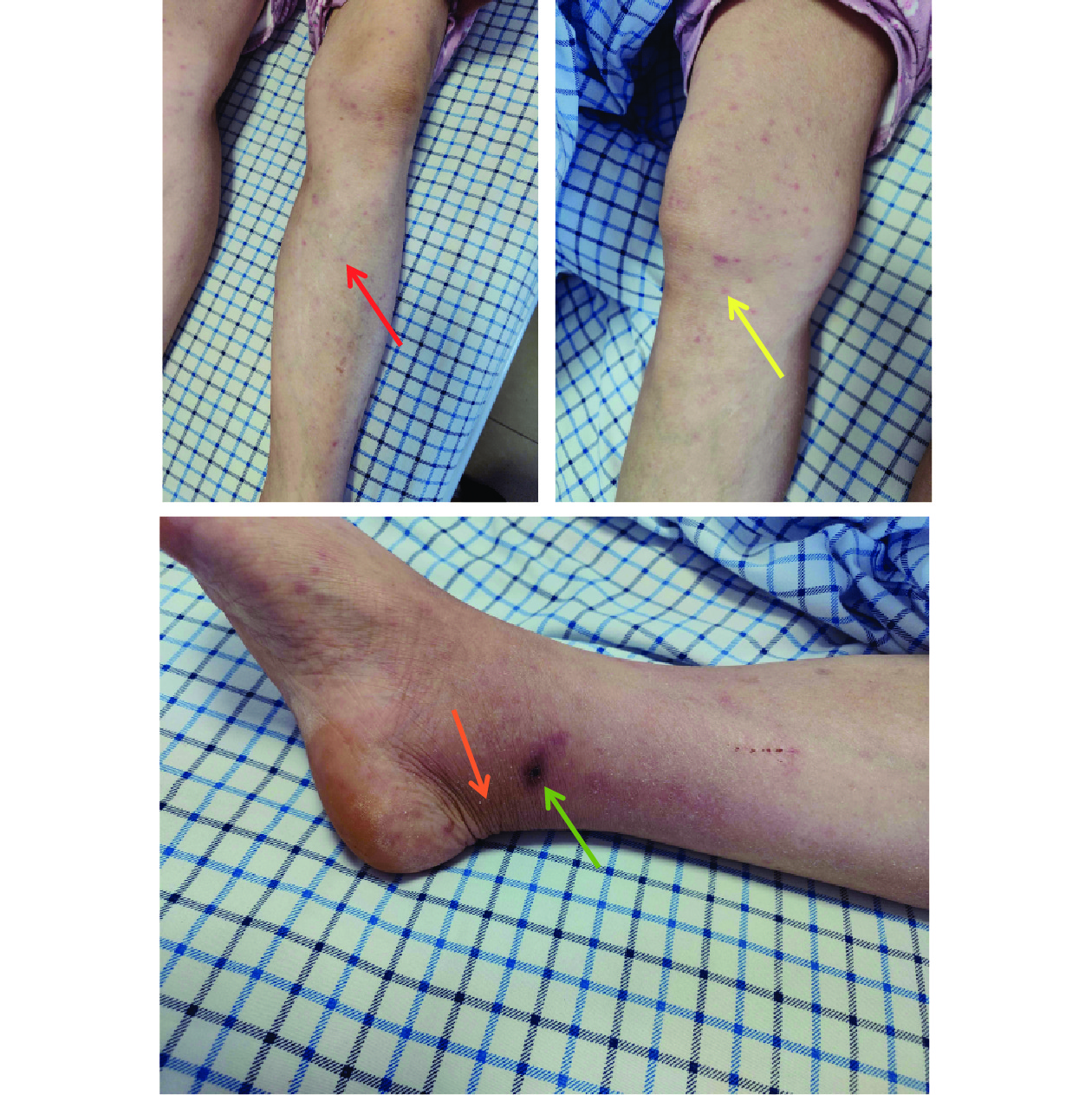 Figure 1.
Figure 1.The changes in the skin of the patient after bite.
Note: Red arrow — early papules; yellow arrow — papules; orange arrow — swelling; green arrow — eschar.Indicator Before treatment After treatment Reference value WBC (109/L) 4.8 7.8 3.5–9.5 NEUT# (109/L) 3.21 4.81 2–7 LYMPH# (109/L) 1.11 2.32 1.1–3.2 MONO# (109/L) 0.51 0.50 0.1–0.6 EO# (109/L) 0.01 0.10 0.02–0.52 BASO# (109/L) 0.00 0.02 0–0.06 NEUT (%) 66.3 62.1 40–75 LYMPH (%) 23.0 29.9 20–50 MONO (%) 10.5 6.5 3–10 EO (%) 0.2 1.3 0.4–0.8 BASO (%) 0.0 0.2 0–1 RBC (1012/L) 5.95 5.43 3.8–5.1 HGB (g/L) 174 156 115–150 HCT (%) 52.6 48.2 35–45 AST (U/L) 75.89 31.56 13–35 ALT (U/L) 60.12 36.46 7–40 GTT (U/L) 51.34 40.46 7–45 Lactate dehydrogenase (U/L) 328.30 280.91 120–250 α-Butyrate dehydrogenase (U/L) 212.04 203.22 72–182 Inguinal lymph node sizes (cm) Right: 2.1×1.1 Left: 2.7×1 Right: 1.2×0.6 Left: 1.5×0.6 − High sensitivity C-reactive protein (mg/L) 25.50 6.22 0–3 PCT (ng/L) 0.54 − <0.05 Note: “−” represents not available.
Abbreviation: WBC=white blood cell; NEUT#=neutrophi; LYMPH#=lymphocyte; MONO#=monocyte; EO#=eosinophil; BASO#=basophil; NEUT%=neutrophil ratio; LYMPH%=lymphocyte ratio; MONO%=monocyte ratio; EO%=eosinophil ratio; BASO%=basophil ratio; RBC=red blood cel; HGB=hemoglobin; HCT=hematocrit; AST=aspartate aminotransferase; ALT=alanine aminotransferase; GTT=γ-Glutamyl transpeptidase; PCT=procalcitonin.Table 1. Biochemical test indicators of the case with scrub typhus.
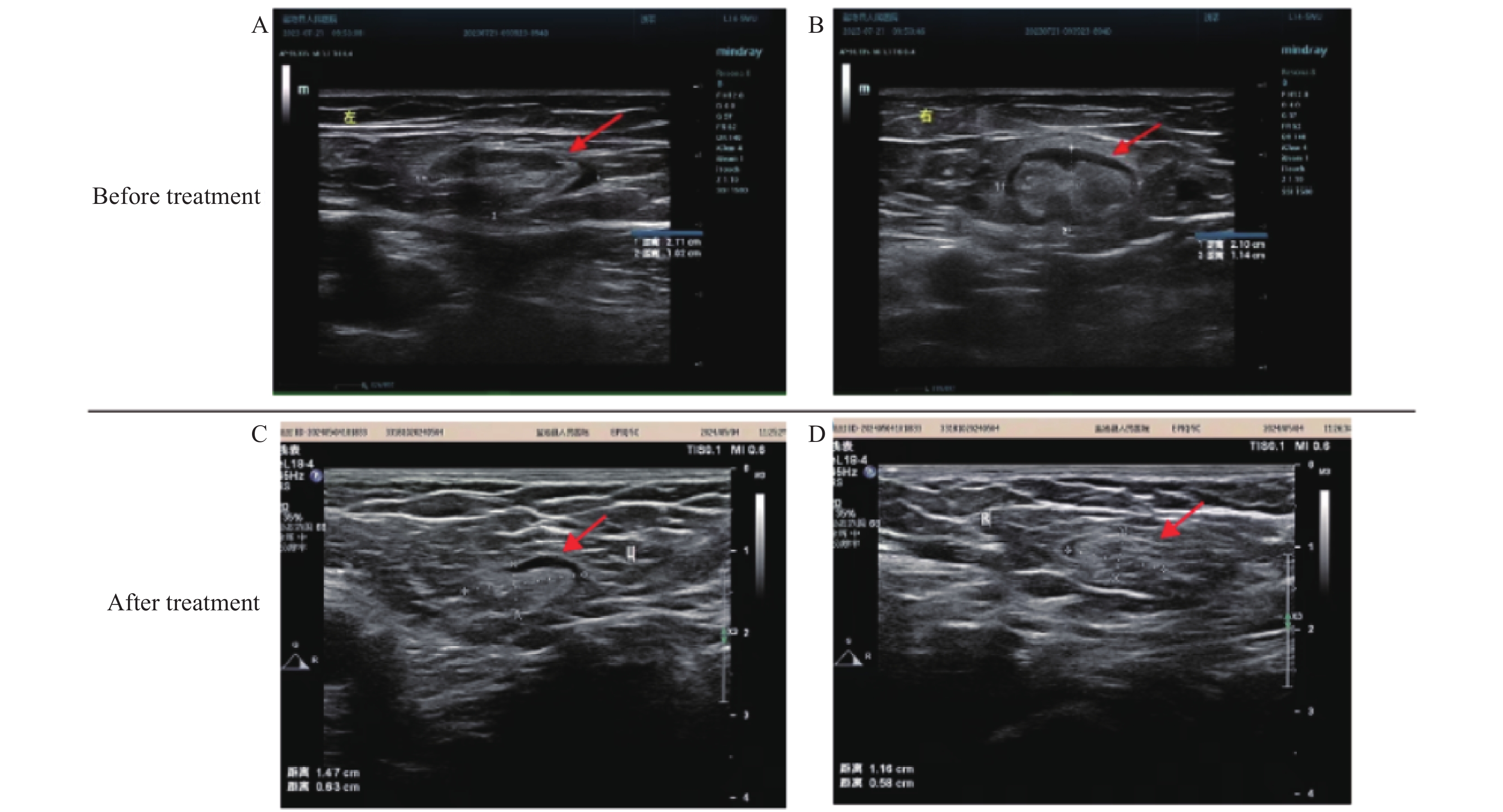 Figure 2.
Figure 2.Computed tomography results of the case. (A) Left and (B) right inguinal lymph nodes before treatment; (C) Left and (D) right inguinal lymph nodes after treatment.
The therapeutic regimen consisted of: 1) intravenous levofloxacin sodium chloride (0.5 g, QD) for anti-infective treatment; 2) intravenous dexamethasone (5 mg) for anti-inflammatory effects; and 3) oral biphenyl diester (50 mg, TID) for hepatoprotection. Follow-up examination on July 28 demonstrated significant clinical improvement, with normalized laboratory parameters and a marked reduction in bilateral inguinal lymph node dimensions (right: 1.2 cm × 0.6 cm; left: 1.5 cm × 0.6 cm) (Table 1 and Figures 2C–D).
Serum samples were collected from both the patient (August 16, 2023) and two local residents serving as controls. All samples underwent qualitative immunoassay analysis using Scrub Typhus DetectTM IgM and IgG ELISA Kits (InBios International, USA). Absorbance measurements at 450 nm were obtained using an ELx808 microplate reader (BioTek, USA). The patient’s serum showed an IgM absorbance of 0.380, compared to control values of 0.068 and 0.067. IgG absorbance values were 0.048 for the patient and 0.023 and 0.036 for the controls.
Environmental investigation of the patient’s residence yielded 33 captured rodents, comprising 12 Mus musculus, 12 Spermophilus alaschanicus, 6 Meriones meridianus, 1 Allactaga sibirica, 1 Cricetulus barabensis, and 1 Rattus norvegicus. From these rodents, 125 fleas and 10 mites were collected. The ectoparasites were homogenized, and nucleic acid was extracted using a Blood & Tissue Kit (69506, Qiagen, Hilden, Germany). Subsequent analysis using an Orientia tsutsugamushi Nucleic Acid Detection Kit (YJBI0145N Fluorescent PCR Assay) yielded negative results.
-
Scrub typhus is an acute infectious disease caused by Orientia tsutsugamushi infection that poses a significant public health challenge in the Asia-Pacific region. While laboratory diagnosis typically relies on serological testing (including Weil-Felix test and ELISA), false-negative results frequently occur during the acute phase. Consequently, diagnosis and treatment decisions often depend on clinical manifestations, which initially present as non-specific influenza-like symptoms (fever, headache, myalgia, and cough), followed by rash and characteristic eschar at the bite site, with potential progression to fatal outcomes in severe cases (4). The non-specific nature of scrub typhus symptoms creates diagnostic challenges, as they overlap with other acute febrile illnesses, including malaria, dengue fever, leptospirosis, and various Rickettsial diseases. The presence of eschar combined with relevant travel or residence history in endemic areas provides crucial diagnostic indicators. The patient presented with characteristic features, including acute onset following insect bite, fever, pruritic white rash, and a black eschar on the medial right ankle, aligning with both the epidemiological history and clinical presentation of scrub typhus, supporting the clinical diagnosis (5). Typical laboratory findings in scrub typhus patients include stable WBC counts with decreased or absent eosinophils, alongside liver function abnormalities marked by elevated AST and ALT levels. The patient’s physical examination, computed tomography findings of bilateral inguinal lymphadenopathy, and blood biochemical profile demonstrated these characteristic features, with positive response to standard therapeutic protocols. While the patient’s acute-phase serology showed positive IgM but negative IgG, similar serological profiles have been documented in previous case reports (3). Despite negative pathogen detection in environmental sampling around the patient’s residence, the absence of travel history within 6 months prior to symptom onset strongly suggests local acquisition. The environment and vectors in the area will be under ongoing surveillance. Without appropriate treatment, scrub typhus can lead to severe complications, including hearing impairment and multiorgan failure, with case fatality rates varying regionally from 30% to 70% (6).
Scrub typhus predominantly occurs in the southwestern, southeastern coastal, and eastern regions of China, though its geographical distribution has recently expanded into northern regions (7). The disease exhibits distinct seasonality, emerging in May and reaching peak incidence during June and July, corresponding to weather patterns and mite life cycles. Agricultural workers constitute a significant proportion of cases (8). The present case aligns with this epidemiological pattern, involving a farmer engaged in crop cultivation whose primary work environment encompasses farmland and grasslands. The patient’s residential circumstances — maintaining livestock near her home amid a high density of rodent populations — created elevated risk conditions for exposure to infected chigger mites. Environmental investigation of the patient’s living space serves multiple crucial purposes: tracing potential epidemiologic exposures, identifying possible pathogen reservoirs, and evaluating how rodent density in residential areas influences Orientia tsutsugamushi transmission. These insights are essential for developing targeted prevention and control strategies.
Currently, significant challenges in controlling scrub typhus include diagnostic delays and misdiagnoses, primarily due to insufficient clinician awareness. In this case, the patient’s delayed healthcare-seeking behavior, combined with the limited diagnostic capabilities at the county hospital, prevented the collection of blood and eschar skin swab specimens for qPCR testing during the acute phase. Furthermore, the absence of local immunological testing facilities and standardized reagents, coupled with healthcare providers’ limited awareness of scrub typhus, resulted in the failure to promptly report the case to the local CDC. Therefore, enhancing the detection and diagnostic capabilities of medical personnel is crucial. Physicians should synthesize laboratory findings with clinical manifestations and immediately report any suspected or confirmed cases of scrub typhus to the CDC, enabling rapid implementation of prevention and control measures to minimize transmission risk.
As the first reported case of scrub typhus in Ningxia Hui Autonomous Region, enhanced surveillance and preventive measures are crucial. While various antibiotics are available for treatment, antimicrobial resistance remains a significant concern (9). Randomized clinical trials have demonstrated comparable efficacy among tetracycline, doxycycline, telithromycin, and azithromycin for treating scrub typhus (10). Azithromycin is particularly recommended due to its efficacy profile comparable to doxycycline (11). The World Health Organization (WHO) specifically recommends azithromycin or chloramphenicol for treating pregnant women and children due to their superior safety profiles. Ongoing research into drug resistance mechanisms is essential for developing new therapeutic strategies and understanding both treatment failures and pathogen resistance patterns. The absence of vaccines for Rickettsial diseases, including scrub typhus, presents a significant challenge, particularly given the substantial antigenic variation among Orientia tsutsugamushi strains, resulting in weak and transient cross-protection. This variation poses considerable obstacles to effective vaccine development. Successful management of Rickettsial diseases requires both prompt initiation of treatment upon clinical suspicion and implementation of robust preventive measures, especially for individuals in or traveling to endemic areas.
-
Approved by the ethics committee of the National Institute for Communicable Disease Control and Prevention of the Chinese Center for Disease Control and Prevention. Verbal informed consent was obtained from the patient.
HTML
| Citation: |



 Download:
Download:




