-
As of December 31, 2023, China reported approximately 1.29 million individuals living with human immunodeficiency virus (HIV)/ acquired immune deficiency syndrome (AIDS), including 719,000 HIV-positive individuals and 570,000 AIDS patients, with 458,000 reported deaths (1). While HIV/AIDS prevalence in China remains low, prevention and control efforts have entered a new phase. A cost-effectiveness analysis funded by the National Natural Science Foundation revealed that regions with substantial financial investment and high antiretroviral therapy coverage are experiencing diminishing marginal returns from existing interventions, suggesting current strategies may be approaching their effectiveness threshold (2-3). This necessitates the exploration of novel and more effective intervention strategies.
Pre-exposure prophylaxis (PrEP) has emerged as a proven HIV prevention method, demonstrating over 90% effectiveness when properly administered (4). PrEP has gained international recognition as a crucial HIV prevention tool, with World Health Organization (WHO) guidelines from 2016 recommending its use for high-risk populations, including men who have sex with men (MSM), female sex workers (FSW), seronegative partners among HIV serodiscordant couples (SNP), and transgender women (5). China’s HIV/AIDS prevention and control frameworks have incorporated PrEP as a key preventive measure (6). Implementation of PrEP in China began in 2017 with Tianjin’s pilot program, expanding to Beijing, Hunan, Yunnan, and Heilongjiang provinces from 2018 to 2019 (7). A significant milestone was reached in August 2020 when the China Food and Drug Administration approved Truvada as the country’s first PrEP medication for HIV prevention in uninfected individuals. The subsequent publication of the Chinese Expert Consensus on HIV Pre-Exposure Prophylaxis Medication in November 2020 provided clinical guidance. However, PrEP implementation faces multiple challenges, including limited acceptance among target populations, incomplete policy frameworks and guidelines specific to the Chinese context, inadequate institutional capacity for PrEP service delivery, and high medication costs.
To address these challenges, the Chinese Association of STD & AIDS Prevention and Control and the National Center for AIDS/STD Control and Prevention jointly initiated the “HIV PrEP Mode Exploration Project” across 24 cities from February 2022 to February 2023. The project aimed to develop and evaluate PrEP implementation models, establish effective service systems, create successful models for PrEP implementation, and generate evidence for updating and enhancing PrEP guidelines.
-
In December 2021, nearly 400 participants from CDCs, non-government organizations (NGOs), and medical institutions across 24 cities attended a comprehensive project training conference. The conference focused on establishing proficiency in project mechanisms, technical guidelines, and data management procedures, while facilitating the development of locally tailored implementation strategies. By February 2022, three distinct PrEP implementation models were established and operational processes were finalized, allowing each city and municipality to select and adapt a model according to their specific circumstances.
-
PrEP Clinics: Dedicated PrEP clinics were established to provide comprehensive services, including consultation, risk assessment, testing, medication dispensing, and follow-up care. The service delivery process encompasses six key components: 1) Scientific outreach: disseminating PrEP knowledge through multiple channels to enhance awareness and demand among target populations; 2) Consultation and referral: providing consultation services through CDC staff, community organizations, or medical institutions, with appropriate referral pathways; 3) Risk assessment: professional medical evaluation of HIV infection risk to determine PrEP eligibility; 4) Medical evaluation: comprehensive screening including HIV, sexually transmitted infections (STI), and liver and kidney function tests to exclude contraindications; 5) Medication initiation: following informed consent, qualified individuals receive prescriptions and obtain PrEP medication from clinic pharmacies; 6) Follow-up care: implementation of routine HIV testing and clinical monitoring to assess medication efficacy and safety, with protocol adjustments as needed.
Digital services and physical testing: This model integrates internet-based medical platforms to deliver comprehensive assessment and medication services. The implementation process encompasses: 1) Health education: dissemination of PrEP information through internet platforms and social media channels; 2) online consultation and risk assessment: Users receive professional consultation through digital platforms; 3) Laboratory testing: based on online physician assessment, individuals either visit medical facilities for testing or utilize self-test kits; 4) digital prescription and delivery: Following prescription confirmation and payment through the online platform, medications are delivered directly to users; 5) telemedicine follow-up: Regular online monitoring with medication regimen adjustments as clinically indicated.
PrEP Self-service Vending Machine: This innovative approach utilizes automated dispensing systems for PrEP medication and HIV self-test kit distribution, primarily targeting experienced PrEP users. The service workflow consists of: 1) QR code-based user registration and information submission; 2) Integration with telemedicine platforms for consultation and risk assessment; 3) Online prescription services based on clinical evaluation results; 4) Automated dispensing of testing and medication packages with remote pharmacist support and follow-up care.
-
Project evaluation incorporated multiple methodologies including on-site assessments, quantitative data analysis, expert panel reviews, and experience-sharing forums. In March 2023, comprehensive project reports were compiled, documenting objectives, implementation phases, outcomes, and key insights to identify best practices. These findings were subsequently disseminated through peer learning sessions, professional meetings, formal reports, and digital platforms.
-
The project successfully established 59 PrEP clinics across 24 cities, primarily integrated into existing antiviral treatment facilities. Eighteen cities implemented collaborative partnerships with internet medical platforms, including BlueCity, JD Health, and DDMedicine. To ensure service quality, the project conducted over 64 capacity-building activities, including meetings, training sessions, and supervisory visits, reaching approximately 2,100 healthcare professionals with comprehensive PrEP-related training.
-
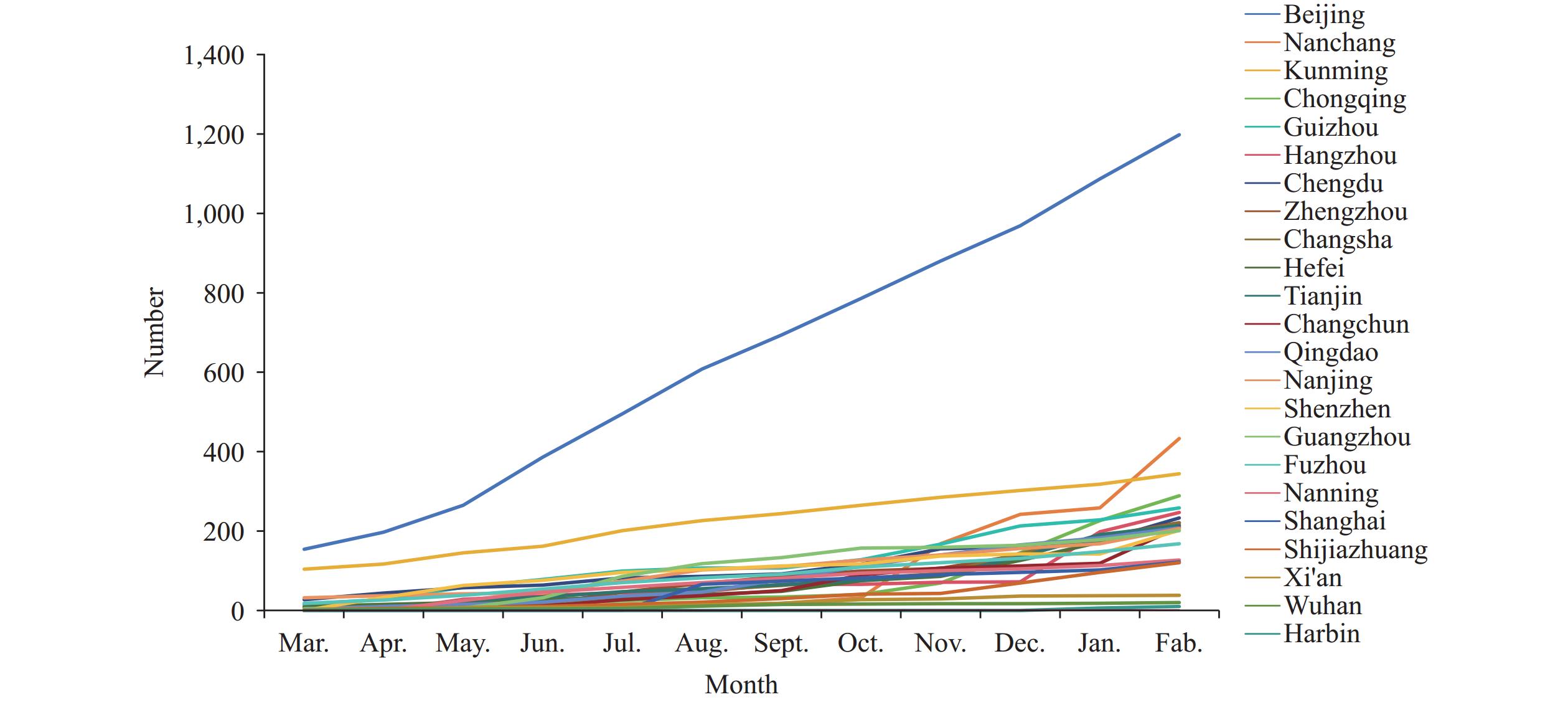
Throughout the project period, HIV testing services were provided to 13,044 individuals. The total number of PrEP users reached 5,505, showing consistent growth over time (Figure 1). The cumulative distribution analysis revealed sustained user growth particularly in Beijing Municipality and Kunming City, Yunnan Province, China (Figure 2). The demographic analysis showed that MSM comprised 97.7% (5,381/5,505) of users, followed by 78 SNP and 46 individuals reporting multiple sexual partnerships.
-
Analysis of advantages and disadvantages: The PrEP clinic model offers distinct advantages in service delivery. First, specialized medical practitioners ensure high-quality care through direct clinical assessments, comprehensive testing, and supervised medication provision. Second, the outpatient service structure maintains complete medical records, facilitating systematic follow-up visits and robust data collection. However, this model presents certain limitations, including potential privacy concerns among clients visiting clinics and a relatively complex service process involving multiple steps such as registration, payment, and waiting times.
The digital services with physical testing model has successfully mitigated concerns regarding discrimination and privacy exposure. This approach offers flexible service timing that aligns with contemporary consumption patterns and enables broad geographical coverage with minimal professional staffing requirements. However, this model faces significant challenges in maintaining consistent follow-up care and comprehensive data collection.
The PrEP self-service vending machine model similarly addresses discrimination and privacy concerns while specifically catering to experienced PrEP users. The primary challenges include developing sophisticated hardware and software systems for automated dispensing machines and ensuring seamless integration with online medical platforms. This model shows potential for widespread adoption once PrEP becomes established as a routine HIV prevention method, similar to the current ubiquity of condom vending machines.
-
Based on their PrEP access patterns for initial and subsequent visits, users were categorized into three distinct groups: those receiving services entirely offline, those transitioning from initial offline to subsequent online services, and those utilizing fully remote consultation and medication delivery. The offline-only group comprised 2,195 individuals, averaging 56 patients per clinic. The hybrid offline-to-online group included 1,754 users, while the fully remote service group consisted of 1,556 users.
Due to requirements for medical assessment and testing, most users (70.73%) initially accessed PrEP through clinics to ensure comprehensive medical evaluation. Subsequently, 60.13% of users transitioned to online services, potentially driven by privacy considerations or coronavirus disease 2019 (COVID-19)-related constraints.
-
Cooperation and expansion: Through strategic partnerships with government agencies, NGOs, healthcare providers, and online medical platforms, we established a comprehensive service network that enhanced PrEP accessibility. This collaborative approach optimized resource allocation through clear role delineation: CDCs coordinated operations, provided technical guidance, and managed data collection; medical institutions conducted promotion activities, medical assessments, medication management, and follow-up care; and NGOs facilitated outreach, counseling, referrals, and retention services, effectively bridging the gap between clients and healthcare providers. This integrated approach not only advanced organizational knowledge and strategy implementation but also fostered the development of innovative PrEP service delivery models.
Optimization of the service process: The service delivery framework encompasses six essential stages: promotion, consultation, assessment, testing, medication dispensing, and follow-up monitoring. While most stages can be conducted either online or offline, renal function testing remains exclusively hospital-based. Cities implemented locally adapted service processes while maintaining standardized training protocols to ensure consistent service quality. The online platform features a streamlined, user-friendly navigation process that prioritizes privacy protection. The offline clinic model, operating in partnership with social organizations, has achieved higher user trust levels through its community-based approach.
-
The awareness and acceptance of PrEP among high-risk populations beyond MSM remains significantly limited. Survey results from participating project cities revealed that among individuals involved in sex work and drug use, only 37.0% had heard of PrEP, and merely 27.3% expressed willingness to use it — markedly lower than the corresponding rates of 86.3% and 96.4% among MSM populations.
Through user interviews regarding PrEP hesitancy, two primary concerns emerged. First, participants expressed apprehension about medication efficacy and adverse effects, as illustrated by one respondent: “My only concern is the side effects, and for me, the side effects are noticeable, such as dizziness.” Second, users feared potential stigmatization associated with PrEP use, exemplified by another participant’s statement: “From what I understand, only those who frequently go to bars need such things. If I take this medication, others might think I’m ‘promiscuous’ and that scares me.” These barriers to PrEP adoption align with previous research findings, which have identified concerns about personal privacy disclosure, medication side effects, and social discrimination as key deterrents (8).
-
The consistent increase in PrEP users throughout the project demonstrates growing recognition of PrEP’s vital role in HIV prevention and heightened adoption willingness. This trend necessitates ensuring stable and accessible drug supply channels. Surveys across project sites identified medication and testing costs as the primary barriers to PrEP adoption, aligning with ZHANG’s findings (9). The limited availability of PrEP medications in China, with only Truvada currently approved, combined with regulatory constraints and pricing issues, has led some users to seek generic alternatives (10).
Qualitative data from MSM participants highlighted these challenges: “Despite the current convenience of medication purchase through applications, many have discontinued their use. Some individuals, particularly those with lower educational levels, lack knowledge about medication access channels.” “Regarding costs, the current pricing structure remains prohibitive. At present rates, I find it financially burdensome.”
-
Initial follow-up evaluations are required within the first month of PrEP initiation to assess HIV status and monitor for adverse reactions. Subsequent follow-up visits are recommended quarterly for HIV and STI screening (11). Analysis of daily PrEP users in Beijing revealed that while initial follow-up achieved 100% coverage, adherence to follow-up visits declined significantly over time. The overall follow-up rate was notably low at 19.47% (Table 1).
Months Initiated PrEP count Scheduled follow-ups Actual follow-ups Follow-up rate (%)* March 59 − − − April 16 59 59 100.00 May 19 16 16 100.00 June 98 19 19 100.00 July 75 157 32 20.38 August 88 91 45 49.45 September 124 107 49 45.79 October 138 281 42 14.95 November 146 229 36 15.72 December 245 253 31 12.25 January 164 526 30 5.70 February 144 393 56 14.25 Total 1,316 2,131 415 19.47 Note: “-” means the first month was not scheduled for follow-up.
Abbreviation: PrEP=pre-exposure prophylaxis.
* The numerator is the number of actual follow-ups, and the denominator is the number of scheduled follow-ups.Table 1. Follow-up status of daily PrEP users in Beijing.
-
The implementation of PrEP in China remains in an exploratory phase, requiring strategic efforts to facilitate its integration into routine HIV prevention services. Three key areas demand immediate attention. First, awareness campaigns need to be expanded beyond MSM to reach other high-risk populations, particularly seronegative partners in HIV-discordant couples and female sex workers. Second, enhanced social support mechanisms and legal frameworks are essential, with specific focus on reducing financial barriers through insurance coverage or government procurement programs to make PrEP accessible to a broader spectrum of high-risk individuals. Third, medical services must incorporate comprehensive psychosocial support to address users’ emotional needs and build trust, thereby improving long-term engagement and follow-up adherence. As these conditions evolve and systems mature, PrEP is positioned to become a crucial component of China’s HIV prevention strategy for key populations.
HTML
Launch Stage
Model Exploration Stage
Evaluation and Promotion Stage
Establishment of PrEP Service Network
Growth in the Number of PrEP Users
Comparison of Three PrEP Implementation Models
Data Comparison
The Refinement of Best Practices
Cognition and Acceptance Remained Low
Drug Accessibility and Prices
Follow-up Management
| Citation: |




 Download:
Download: