-
Antiretroviral therapy (ART) can dramatically reduce the human immunodeficiency virus (HIV) viral load (VL) in people living with HIV (PLHIV). However, a subset of PLHIV experience detectable VL between 50 and 999 copies/ml during long-term ART, a phenomenon referred to as low-level viremia (LLV) (1). Multiple studies have demonstrated that LLV is associated with adverse clinical outcomes, including virological failure (1-5). Despite these findings, the relationship between first-year LLV and subsequent VL trajectories remains understudied in China. This study investigated the association between first-year LLV and subsequent viral non-suppression during five-year follow-ups in Dehong Dai and Jingpo Autonomous Prefecture, Yunnan Province, China. We analyzed data from 4,087 PLHIV on ART who achieved first-year VL <1,000 copies/mL in Dehong Prefecture from 2008 to 2021, employing nominal logistic regression analyses to examine these associations. Among the study population, 12.8% experienced first-year LLV of 50–199 copies/mL, while 4.8% had LLV of 200–999 copies/mL. The adjusted odds ratios (aORs) [95% confidence interval (CI)] of first-year LLV for five-year LLV 50–999 and VL ≥1,000 copies/mL were 18.99 (95% CI: 14.55, 24.79) and 4.90 (95% CI: 3.55, 6.76), respectively. Our findings demonstrate that first-year LLV of 50–999 copies/mL significantly increases the risk of poor virological outcomes, highlighting the critical need for enhanced monitoring and intervention strategies for early LLV occurrence in patients on ART.
We conducted a retrospective cohort study analyzing treatment-naive PLHIV enrolled in China’s National Free Antiretroviral Treatment Program in Dehong Prefecture, Yunnan Province, Southwest China. Dehong Prefecture is a highly endemic area for HIV transmission and holds historical significance as the location of China’s first reported HIV infection through injection drug use (IDU) in 1989. Well-trained local healthcare providers collected comprehensive data through participant interviews, including demographic characteristics, HIV infection details, VL measurements, CD4 cell counts, hemoglobin levels, hepatitis B surface antigen (HBsAg), anti-hepatitis C virus (HCV) antibody status, ART regimens, initiation dates, and adherence (6–7). Bad ART adherence was defined as ≥2 missed ART doses during first-year or two-year follow-ups, or ≥3 missed doses during five-year follow-ups. Follow-up duration was segmented into one-year intervals after six months of ART: 1±0.5, 2±0.5, 3±0.5, 4±0.5, and 5±0.5 years. For multiple VL tests within an interval, the last value was used. Study inclusion criteria were: 1) ART initiation in Dehong Prefecture between 2008 and 2016 with follow-up through 5 years or until December 2021; 2) age ≥18 years at ART initiation; 3) minimum of three VL tests after 6 months of ART initiation; and 4) first-year VL <1,000 copies/mL (achieving virological success) with documented second-year VL. Participants were excluded if their transmission route was neither sexual contact nor IDU, or if ethnicity, education level, or baseline CD4 data were missing.
We analyzed participants’ baseline characteristics stratified by first-year VL groups and described VL distribution (VL <50, LLV 50–199, LLV 200–999, and ≥1,000 copies/mL) across follow-up years. Univariate and multivariate nominal logistic regression models using stepwise selection investigated associations between first-year VL and subsequent viral outcomes. Second-year VL outcomes were categorized as <50, LLV 50–999, and ≥1,000 copies/mL. Five-year VL profiles were classified as: VL ≥1,000 (≥2 instances of VL ≥1,000 copies/mL or one VL ≥1,000 copies/mL plus one LLV 50–999), LLV 50–999 (one or no VL ≥1,000 copies/mL and ≥2 instances of LLV 50–999), and VL <50 (maximum one instance of VL ≥50 copies/mL, otherwise <50 copies/mL). Statistical significance was set at P<0.05 (two-tailed), and analyses were performed using SAS (version 9.4; SAS Institute Inc., Cary, NC, USA). The study received approval from the institutional review board of the National Center for AIDS/STD Control and Prevention, Chinese Center for Disease Control and Prevention.
Among 4,087 participants enrolled in Dehong Prefecture, Yunnan Province during 2008–2021, 93.8% (4,087/4,356) achieved first-year virological success. The cohort’s median age was 36 years (interquartile range, IQR: 30–44), with 56.0% being male and 79.2% acquiring HIV through sexual transmission. The ethnic composition comprised Han (44.6%), Dai (29.6%), and Jingpo (20.2%) populations. Educational attainment was predominantly at or below primary school level (58.4%). Baseline median CD4 count was 256 cells/μL (IQR: 145–369 cells/μL), with 36.4% of participants having counts below 200 cells/μL. ART initiation was distributed across three periods: 2008–2010 (30.5%), 2011–2013 (46.9%), and 2014–2016 (22.6%). The standard first-line regimen — [tenofovir (TDF) or zidovudine (AZT)] + lamivudine (3TC) + [efavirenz (EFV) or nevirapine (NVP)] — was initiated in 79.8% of participants. During the first year of ART, 12.8% and 4.8% of participants experienced LLV 50–199 and LLV 200–999 copies/mL, respectively (Table 1). At the five-year follow-up conclusion, treatment retention was 94.36% (3,856 participants), with 2.4% (99) lost to follow-up and 3.2% (132) deceased.
Characteristics Total (%) (N=4,087) VL <50 copies/mL
(n=3,368, 82.4%)LLV 50–199 copies/mL
(n=523, 12.8%)LLV 200–999 copies/mL
(n=196, 4.8%)Age at ART 18–30 years 1,172 (28.7) 976 (83.3) 140 (11.9) 56 (4.8) 31–50 years 2,458 (60.1) 2,004 (81.5) 329 (13.4) 125 (5.1) >50 years 457 (11.2) 388 (84.9) 54 (11.8) 15 (3.3) Gender Female 1,799 (44.0) 1,510 (83.9) 201 (11.2) 88 (4.9) Male 2,288 (56.0) 1,858 (81.2) 322 (14.1) 108 (4.7) Ethnicity Han 1,823 (44.6) 1,476 (81.0) 267 (14.6) 80 (4.4) Dai 1,208 (29.5) 1,010 (83.6) 145 (12.0) 53 (4.4) Jingpo 824 (20.2) 682 (82.8) 93 (11.3) 49 (5.9) Other 232 (5.7) 200 (86.2) 18 (7.8) 14 (6.0) Education Illiteracy 591 (14.5) 508 (86.0) 64 (10.8) 19 (3.2) Primary school 1,795 (43.9) 1,446 (80.6) 230 (12.8) 119 (6.6) Middle school and above 1,701 (41.6) 1,414 (83.1) 229 (13.5) 58 (3.4) Transmission route Sexual contact 3,235 (79.2) 2,699 (83.4) 392 (12.1) 144 (4.5) IDU 852 (20.8) 669 (78.5) 131 (15.4) 52 (6.1) Duration from HIV diagnosis to ART <1 years 2,134 (52.2) 1,740 (81.5) 298 (14) 96 (4.5) ≥1 years 1,953 (47.8) 1,628 (83.4) 225 (11.5) 100 (5.1) WHO clinical stage 1–2 2,283 (55.9) 1,950 (85.4) 242 (10.6) 91 (4.0) 3–4 1,804 (44.1) 1,418 (78.6) 281 (15.6) 105 (5.8) Baseline CD4 cells, cells/μL <200 1,490 (36.4) 1,161 (77.9) 238 (16.0) 91 (6.1) 200–349 1,453 (35.6) 1,158 (79.7) 214 (14.7) 81 (5.6) ≥350 1,144 (28.0) 1,049 (91.7) 71 (6.2) 24 (2.1) Baseline hemoglobin, g/L ≥90 3,847 (94.1) 3,179 (82.6) 490 (12.8) 178 (4.6) <90 192 (4.7) 147 (76.6) 28 (14.6) 17 (8.9) Not tested 48 (1.2) 42 (87.5) 5 (10.4) 1 (2.1) Baseline HBsAg Negative 2,847 (69.6) 2,521 (88.6) 249 (8.7) 77 (2.7) Positive 215 (5.3) 190 (88.4) 22 (10.2) 3 (1.4) Not tested 1,025 (25.1) 657 (64.1) 252 (24.6) 116 (11.3) Baseline Anti-HCV Negative 2,306 (56.4) 2,050 (88.9) 195 (8.5) 61 (2.6) Positive 544 (13.3) 465 (85.5) 64 (11.8) 15 (2.7) Not tested 1,237 (30.3) 853 (69.0) 264 (21.3) 120 (9.7) Baseline regimen TDF+3TC+EFV/NVP 1,427 (34.9) 1,323 (92.7) 77 (5.4) 27 (1.9) AZT+3TC+EFV/NVP 1,833 (44.9) 1,466 (80.0) 274 (14.9) 93 (5.1) LPV/r+3TC+AZT/TDF 316 (7.7) 292 (92.4) 16 (5.1) 8 (2.5) Other* 511 (12.5) 287 (56.2) 156 (30.5) 68 (13.3) ART initiation year 2008–2010 1,246 (30.5) 775 (62.2) 342 (27.4) 129 (10.4) 2011–2013 1,916 (46.9) 1,732 (90.4) 137 (7.1) 47 (2.5) 2014–2016 925 (22.6) 861 (93.1) 44 (4.7) 20 (2.2) Abbreviation: 3TC=lamivudine; ART=antiretroviral therapy; AZT=zidovudine; CD4=CD4+ T lymphocytes; CI=confidence interval; D4T=stavudine; EFV=efavirenz; HBsAg=hepatitis B surface antigen; HCV=hepatitis C virus; HIV=human immunodeficiency virus; IDU=injection drug use; LLV=low-level viremia; LPV/r=lopinavir/ritonavir; NVP=nevirapine; OR=odds ratio; TDF=tenofovir; VL=viral load; VS=viral suppression.
* Primarily D4T+3TC+EFV/NVP regimen (491, 12.0%).Table 1. Baseline characteristics of 4,087 people living with HIV who achieved virological success in first-year ART in Dehong Prefecture, Yunnan Province, China, 2008–2021.
The proportion of participants experiencing LLV decreased progressively over the five-year follow-up period: 17.6% (719/4,087) in year 1, 12.4% (505/4,087) in year 2, 8.1% (291/3,590) in year 3, 6.6% (228/3,436) in year 4, and 5.6% (185/3,329) in year 5 (Figure 1). Second-year viral load distribution showed 83.5% with VL <50 copies/mL, 12.3% with LLV 50–999 copies/mL and 4.2% with VL ≥1,000 copies/mL. The five-year viral load profile revealed 84.6% maintained VL <50 copies/mL, 10.1% experienced LLV, and 5.3% had VL ≥1,000 copies/mL.
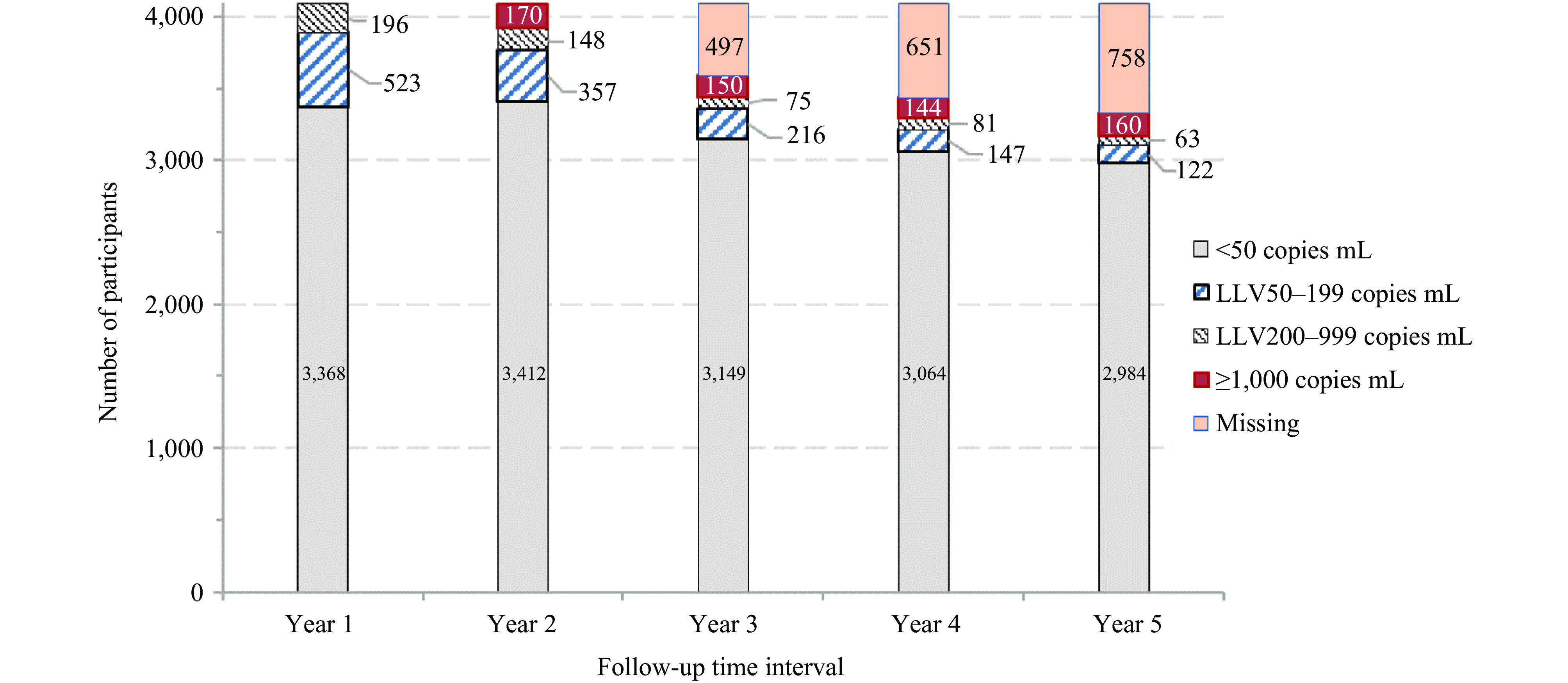 Figure 1.
Figure 1.Temporal distribution of viral load levels during 5 years of antiretroviral therapy among 4,087 people living with HIV who achieved initial virological success in Dehong Prefecture, Yunnan Province, China, 2008–2021.
Note: Missing data indicates the proportion of individuals for whom viral load measurements were insufficient to determine viral load categorization.Using multivariate logistic regression with VL <50 copies/mL as the reference event, participants with first-year LLV showed significantly higher risks of subsequent viral non-suppression. The aORs for second-year outcomes were 2.58 (95% CI: 2.06, 3.24) for LLV 50–999 copies/mL and 3.42 (95% CI: 2.37, 4.93) for VL ≥1,000 copies/mL. For five-year outcomes, the aORs were even more pronounced: 18.99 (95% CI: 14.55, 24.79) for LLV 50–999 copies/mL and 4.90 (95% CI: 3.55, 6.76) for VL ≥1,000 copies/mL (Table 2). The association between first-year LLV and subsequent detectable VL remained robust across multiple sensitivity analyses, accounting for baseline CD4 count, ART initiation year, adherence, and regimen changes.
Covariate* Second-year VL profile Five-year VL profile LLV 50–999 copies/mL VL ≥1,000 copies/mL LLV 50–999 copies/mL VL ≥1,000 copies/mL OR
(95% CI)aOR
(95% CI)OR
(95% CI)aOR
(95% CI)OR
(95% CI)aOR
(95% CI)OR
(95% CI)aOR
(95% CI)First-year VL (copies/mL) VL<50 1.0 (Ref) 1.0 (Ref) 1.0 (Ref) 1.0 (Ref) 1.0 (Ref) 1.0 (Ref) 1.0 (Ref) 1.0 (Ref) LLV 50–999 4.22
(3.44, 5.17)†2.58
(2.06, 3.24)†3.51
(2.52, 4.88)†3.42
(2.37, 4.93)†28.16
(21.94, 36.14)†18.99
(14.55, 24.79)†5.58
(4.14, 7.52)†4.90
(3.55, 6.76)†Transmission route Sexual contact 1.0 (Ref) 1.0 (Ref) 1.0 (Ref) 1.0 (Ref) 1.0 (Ref) 1.0 (Ref) 1.0 (Ref) 1.0 (Ref) IDU 1.11
(0.89, 1.40)0.92
(0.72, 1.19)2.12
(1.53, 2.94)†1.55
(1.09, 2.22)§1.44
(1.14, 1.82)†1.04
(0.75, 1.44)2.31
(1.73, 3.09)†1.74
(1.23, 2.47)†Duration from HIV diagnosis
to ART− − <1 years 1.0 (Ref) 1.0 (Ref) 1.0 (Ref) 1.0 (Ref) 1.0 (Ref) 1.0 (Ref) ≥1 years 0.97
(0.80, 1.17)1.05
(0.85, 1.29)1.67
(1.22, 2.28)†1.57
(1.10, 2.22)§0.94
(0.76, 1.15)1.52
(1.15, 2.01)†WHO clinical stage − − 1–2 1.0 (Ref) 1.0 (Ref) 1.0 (Ref) 1.0 (Ref) 1.0 (Ref) 1.0 (Ref) 3–4 0.72
(0.53, 0.96)1.02
(0.68, 1.52)2.02
(1.64, 2.49)†1.44
(1.10, 1.88)†0.99
(0.75, 1.30)1.05
(0.77, 1.43)Baseline CD4 cells, cells/μL − − <200 2.73
(2.06, 3.61)†1.65
(1.21, 2.24)†1.41
(0.95, 2.09)1.41
(0.92, 2.17)3.59
(2.58, 5.01)†1.72
(1.19, 2.48)†200–349 2.57
(1.94, 3.41)†1.56
(1.15, 2.12)†1.32
(0.88, 1.98)1.28
(0.83, 1.97)3.39
(2.43, 4.74)†1.61
(1.11, 2.33)§≥350 1.0 (Ref) 1.0 (Ref) 1.0 (Ref) 1.0 (Ref) 1.0 (Ref) 1.0 (Ref) Baseline HBsAg Negative 1.0 (Ref) 1.0 (Ref) 1.0 (Ref) 1.0 (Ref) 1.0 (Ref) 1.0 (Ref) 1.0 (Ref) 1.0 (Ref) Positive 1.39
(0.89, 2.17)1.36
(0.86, 2.14)0.54
(0.20, 1.48)0.60
(0.22, 1.65)1.26
(0.71, 2.21)1.35
(0.72, 2.52)1.66
(0.95, 2.89)1.79
(1.01, 3.19)§Not tested 3.80
(3.12, 4.63)†2.37
(1.87, 3.00)†2.34
(1.69, 3.23)†2.42
(1.64, 3.58)†5.80
(4.67, 7.22)†2.57
(1.48, 4.46)†2.22
(1.65, 2.99)†0.91
(0.53, 1.56)Baseline anti-HCV − − Negative 1.0 (Ref) 1.0 (Ref) 1.0 (Ref) 1.0 (Ref) 1.0 (Ref) 1.0 (Ref) Positive 0.89
(0.63, 1.26)1.75
(1.11, 2.78)§1.59
(1.10, 2.30)§1.43
(0.90, 2.27)2.17
(1.47, 3.20)†1.39
(0.87, 2.22)Not tested 3.07
(2.51, 3.75)†2.66
(1.90, 3.74)†4.92
(3.90, 6.20)†1.12
(0.64, 1.99)2.58
(1.90, 3.50)†2.24
(1.31, 3.83)†ART initiation year − − 2008–2010 4.41
(3.28, 5.94)†2.06
(1.47, 2.89)†1.32
(0.86, 2.01)0.58
(0.35, 0.98)§6.14
(4.41, 8.55)†1.90
(1.27, 2.84)†2011–2013 1.42
(1.04, 1.93)§1.49
(1.08, 2.05)§1.00
(0.67, 1.49)1.00
(0.66, 1.51)0.94
(0.65, 1.37)1.31
(0.89, 1.92)2014–2016 1.0 (Ref) 1.0 (Ref) 1.0 (Ref) 1.0 (Ref) 1.0 (Ref) 1.0 (Ref) Baseline regimen − − TDF+3TC+EFV/NVP 1.0 (Ref) 1.0 (Ref) 1.0 (Ref) 1.0 (Ref) 1.0 (Ref) 1.0 (Ref) AZT+3TC+EFV/NVP 1.77
(1.40, 2.25)†1.04
(0.73, 1.49)2.66
(1.97, 3.59)†1.23
(0.87, 1.75)1.32
(0.96, 1.82)1.14
(0.80, 1.62)LPV/r+3TC+AZT/TDF 0.71
(0.42, 1.19)0.77
(0.39, 1.52)1.13
(0.63, 2.01)1.23
(0.66, 2.28)0.85
(0.45, 1.59)0.96
(0.50, 1.83)Other 4.58
(3.47, 6.04)†2.00
(1.27, 3.15)†10.05
(7.27, 13.89)†2.10
(1.37, 3.22)†2.53
(1.67, 3.82)†1.46
(0.90, 2.38)Adherence to ART Good 1.0 (Ref) 1.0 (Ref) 1.0 (Ref) 1.0 (Ref) 1.0 (Ref) 1.0 (Ref) 1.0 (Ref) 1.0 (Ref) Bad 0.91
(0.68, 1.22)1.39
(1.02, 1.90)§2.31
(1.61, 3.34)†2.62
(1.78, 3.85)†1.00
(0.75, 1.34)1.61
(1.13, 2.29)†2.83
(2.10, 3.82)†3.22
(2.32, 4.48)†Abbreviation: aOR=adjusted odds ratio; AIDS=acquired immune deficiency syndrome; ART=antiretroviral therapy; CD4=CD4+ T lymphocytes; CI=confidence interval; HIV=human immunodeficiency virus; LLV=low-level viremia; Ref=reference group; VL=viral load.
* Univariate and multivariate multinomial logistic regression analyses were conducted separately for second-year VL and five-year VL profile models, with VL <50 copies/mL serving as the reference outcome. Sex, ethnicity, and education were statistically significant in univariate analyses (P<0.10) but not in multivariate analyses (P≥0.05) and are therefore not shown. Age and hemoglobin were not significant in univariate analyses (P≥0.10).
† P<0.01;
§ P<0.05.Table 2. Associations of first-year low-level viremia with second-year and five-year viral load profiles among 4,087 adult people living with HIV on antiretroviral therapy with virological success in Dehong Prefecture, Yunnan Province, China, 2008–2021.
-
This cohort study, comprising 4,087 participants on ART from 2008–2021 in Dehong Prefecture, demonstrated that 17.6% of participants experienced LLV of 50–999 copies/mL during their first year of ART follow-up. Furthermore, the presence of first-year LLV significantly increased the risk of sustained LLV or VL ≥1,000 copies/mL during early ART follow-up.
The observed 17.6% prevalence of first-year LLV among participants achieving virological success in Dehong Prefecture (2008–2021), along with the 10.1% five-year LLV rate, aligns with findings reported by Hermans, et al. (1), An, et al. (8), Ding, et al. (9), and Bai, et al. (2). These consistent findings across studies indicate that LLV represents a common challenge in ART management among PLHIV in Dehong Prefecture, warranting careful consideration in treatment protocols.
The multivariate logistic model demonstrated that first-year LLV of 50–999 copies/mL was significantly associated with both second-year VL and five-year viral non-suppression, predicting subsequent sustained LLV or VL ≥1,000 copies/mL. These findings align with previous research (1,8). Various factors, including ART initiation year and baseline regimen (3,10), may contribute to the development of subsequent LLV or virological failure. Drug resistance rates among PLHIV with LLV vary considerably, ranging from 10% to 40%. Current protocols in China mandate adherence assessment and drug-resistance testing when PLHIV experience persistent VL ≥1,000 copies/mL during ART. Since 2022, Dehong Prefecture has implemented a case-management program targeting PLHIV with LLV 200–999 copies/mL. Given the significance for sustained virological success, monitoring and interventions for early-stage LLV 50–999 copies/mL should be piloted and expanded.
This study has several limitations. First, as the data are derived from a single city in Yunnan Province, China, generalizability to other regions require caution. Second, the use of routine clinical data, with only one VL measurement per follow-up year, may introduce selection and information bias through data entry processes.
In conclusion, analysis of this PLHIV cohort on ART from Dehong Prefecture, Southwest China (2008–2021) revealed that first-year LLV of 50–999 copies/mL significantly increased the risk of poor virological outcomes. These findings underscore the importance of early LLV monitoring in ART patients and the need for timely interventions when detected.
-
The healthcare staff for their dedicated support in HIV/AIDS prevention and control efforts in Dehong Prefecture and Dr. Zunyou Wu, who deceased on 27 October 2023, for his substantial contributions to this work.
HTML
| Citation: |



 Download:
Download:




