-
On July 15, 2024, two patients suspected of foodborne botulism presented to the Emergency Department of Weihai Municipal Hospital in Shandong Province, China. The patients, who had ingested homemade pickled eggs, presented with symptoms such as foot/wrist drop, difficulty breathing, diplopia, and ptosis. The Huancui District CDC and Weihai CDC immediately initiated epidemiological investigations, C. botulinum toxin detection, strain isolation, and molecular identification. Based on the epidemiological investigation, laboratory testing, and toxicological test results, this event was determined to be an outbreak of foodborne botulism caused by consumption of homemade pickled eggs contaminated with C. botulinum.
-
At 07:01 and 09:39 on July 15, Weihai Municipal Hospital admitted two severely ill patients (Patient A and B) presenting with identical symptoms, including fatigue, foot/wrist drop, nausea, shortness of breath, chest tightness, dyspnea, paralysis, speech difficulties, blurred vision, diplopia, and ptosis (Figure 1). Both patients had consumed homemade pickled eggs. The hospital made a preliminary diagnosis of a foodborne disease outbreak and promptly reported it to the Huancui District CDC. The Huancui District CDC arrived at the hospital at 12:00 to conduct an epidemiological investigation, focusing on the source of the pickled eggs and other individuals who had consumed them. Preliminary judgments indicated a suspected outbreak of foodborne disease caused by botulinum toxin in contaminated pickled eggs. The initial epidemiological investigation report on the incident was submitted to the Huancui District Health Commission and Weihai CDC at 14:30.
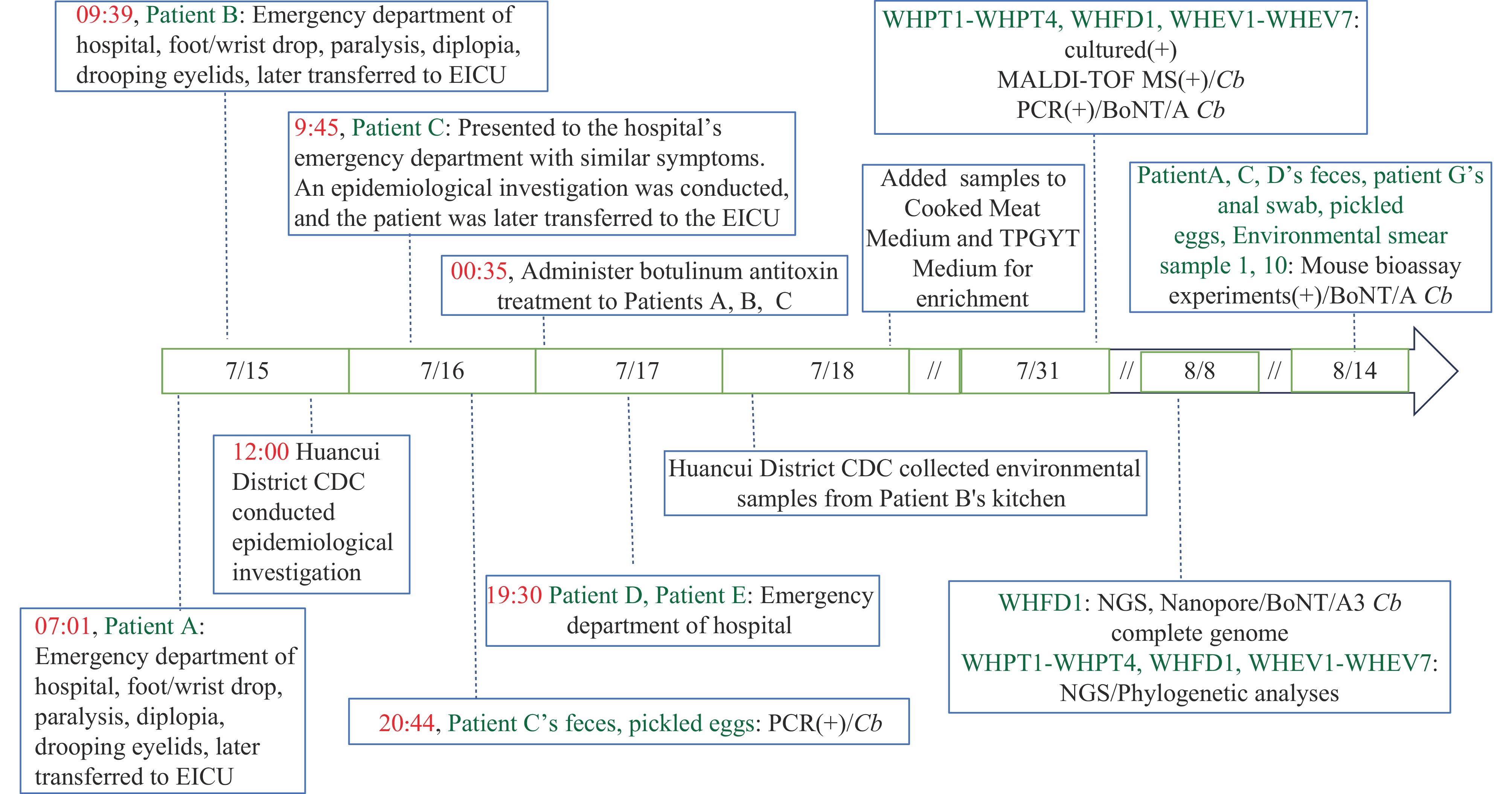 Figure 1.
Figure 1.Timeline of symptom onset, epidemiological investigation, and pathogen identification in patients.
Abbreviation: EICU=emergency intensive care unit; Cb=Clostridium botulinum; MALDI-TOF MS=Matrix-Assisted Laser Desorption/Ionization Time of Flight Mass Spectrometry.From July 16 to 17, the hospital admitted three additional patients (Patients C, D, and E) with the same food exposure history and similar symptoms. The Huancui District CDC investigated the incident. Epidemiological investigation revealed Patient B prepared pickled eggs using home-raised chicken eggs in late June 2024. The eggs, laid by free-range hens in the village, were washed, boiled, cooled, and brined with edible salt at room temperature for approximately two weeks. From July 12 to 14, Patient B gave portions of the pickled eggs to her son (Patient D) and daughter-in-law (Patient C), who then shared them with colleagues (Patients A, E, F, G, and H). A total of eight individuals consumed the pickled eggs without heating (Table 1) and subsequently presented with varying degrees of similar symptoms.
Patients Sex Age (years) Eating time Amount of eggs consumed Onset time Patient A Female 43 07:00, July 13 1 08:00, July 13 11:30, July 12 1 10:00, July 13 Patient B Female 57 17:30, July 12 2 10:00, July 13 Patient C Female 38 11:30, July 12 1 07:00, July 15 11:30, July 13 1 17:30, July 14 1 Patient D Male 39 17:30, July 14 1 13:00, July 16 Patient E Female 56 11:30, July 13 1/2 20:00, July 17 Asymptomatic patient F Male 31 11:30, July 13 1 − Asymptomatic patient G Female 23 11:30, July 13 1/4 − Asymptomatic patient H Female 54 11:30, July 13 1/2 − Note: “−” means no symptoms have appeared. Table 1. Basic information of individuals who consumed the pickled eggs, including eating habits and disease conditions — Weihai City, China, July 2024.
On July 16, clinical samples (Patient A’s enema solution and Patient C’s feces) and pickled eggs collected from the hospital were sent to Weihai CDC for laboratory testing and analysis. Quantitative polymerase chain reaction (qPCR) detection was performed on the clinical samples and pickled eggs using the nucleic acid detection reagent kit for Clostridium botulinum (types A, B, E, and F). Patient C’s feces and pickled eggs tested positive for C. botulinum BoNT/A (Table 2).
Source qPCR before enrichment Enrichment in cooked meat medium and TPGYT medium qPCR after enrichment Colony morphology on the blood agar MALDI-TOF MS isolated strain WGS Mouse bioassay experiments Patient A (feces) NEG Digestion of meat particles BoNT/A Flat, spreading growth pattern C. botulinum WHPT1 BoNT/A3 BoNT/A Patient C (feces) BoNT/A Digestion of meat particles BoNT/A Flat, spreading growth pattern C. botulinum WHPT2 BoNT/A3 BoNT/A Patient D (feces) NEG Digestion of meat particles BoNT/A Flat, spreading growth pattern C. botulinum WHPT3 BoNT/A3 BoNT/A Patient E (feces) NEG Digestion of meat particles NEG ND ND ND ND ND Asymptomatic Patient F (Anal swab) NEG Digestion of meat particles NEG ND ND ND ND ND Asymptomatic Patient G (Anal swab) NEG Digestion of meat particles BoNT/A Flat, spreading growth pattern C. botulinum WHPT4 BoNT/A3 BoNT/A Asymptomatic Patient H (Anal swab) NEG Digestion of meat particles NEG ND ND ND ND ND pickled eggs BoNT/A Digestion of meat particles BoNT/A Flat, spreading growth pattern C. botulinum WHFD1 BoNT/A3 BoNT/A EV 1 (raw eggs) ND Digestion of meat particles BoNT/A Flat, spreading growth pattern C. botulinum WHEV1 BoNT/A3 BoNT/A EV 2 (Inner pot of rice cooker) ND No digestion of meat particles NEG ND ND ND ND ND EV 3 (refrigerator) ND Digestion of meat particles BoNT/A Flat, spreading growth pattern C. botulinum WHEV2 BoNT/A3 ND EV 4 (Sink) ND No digestion of meat particles NEG ND ND ND ND ND EV 5 (pot) ND Digestion of meat particles BoNT/A Flat, spreading growth pattern C. botulinum WHEV3 BoNT/A3 ND EV6 (kitchen cupboards) ND No digestion of meat particles NEG ND ND ND ND ND EV 7 (Salt jar) ND Digestion of meat particles BoNT/A Flat, spreading growth pattern C. botulinum WHEV4 BoNT/A3 ND EV 8 (casserole) ND Digestion of meat particles BoNT/A Flat, spreading growth pattern C. botulinum WHEV5 BoNT/A3 ND EV 9 (Dining plate) ND Digestion of meat particles BoNT/A Flat, spreading growth pattern C. botulinum WHEV6 BoNT/A3 ND EV 10 (garbage bin) ND Digestion of meat particles BoNT/A Flat, spreading growth pattern C. botulinum WHEV7 BoNT/A3 BoNT/A Abbreviation: qPCR=quantitative polymerasechain reaction; EV=environmental smear sample; ND=not detected; NEG=negative. Table 2. qPCR, enrichment, isolation, MALDI-TOF MS, WGS, and mouse bioassay experiments of samples collected in this study — Weihai City, China, July 2024.
At 00:35 on July 17, based on the symptoms and qPCR results, the hospital administered botulinum antitoxin treatment. By July 18, three patients (Patients A, B, and C) were being treated in the Emergency Intensive Care Unit (EICU) of Weihai Municipal Hospital. Two patients (Patients D and E) had mild symptoms; no deaths occurred.
On July 18, to identify and trace the outbreak source, the Huancui District CDC collected 10 environmental samples from Patient B’s kitchen and refrigerator. These samples included smears of raw eggs, kitchen utensils used for storing and cleaning pickled eggs, and swabs from the refrigerator and garbage bin (Table 2).
A total of 18 samples, including clinical, pickled egg, and environmental samples collected from July 16 to 18, were processed for enrichment culture and strain isolation according to GB 4789.12-2016 (1). Samples were added to Cooked Meat Medium and TPGYT Medium and anaerobically incubated at 36 ℃ and 28 ℃, respectively, for 10 days to enrich for C. botulinum. A loopful of enrichment broth that tested positive for the toxin gene by qPCR was streaked onto blood agar and anaerobically incubated at 36 ℃ for 24 hours. Suspected colonies exhibiting flat, smooth, spreading growth with irregular edges were subcultured on blood agar and further identified using qPCR and the Microflex MALDI-TOF MS system (Bruker, Germany).
On July 31, based on qPCR and MALDI-TOF MS results, 12 strains of botulinum bacteria were isolated from the pickled eggs, rectal swabs/feces of patients, and environmental specimens (including smears from plates, sinks, and trash cans). A total of 7 enrichment cultures were selected and sent to the China CDC for mouse bioassay experiments. On August 14, the mouse bioassay showed that all 7 enrichment cultures contained botulinum neurotoxin type A (BoNT/A) (Table 2).
On August 8, we performed sequencing of 12 C. botulinum strains isolated in this study. Additionally, 67 complete C. botulinum genome sequences were downloaded from the GenBank database. Snippy (v4.6.0, Melbourne, Australia) was used to perform core genome single nucleotide polymorphism (SNP) calling and phylogenetic tree construction on the 12 C. botulinum isolates and 67 C. botulinum genome sequences. Phylogenetic analysis revealed that the 12 C. botulinum strains isolated in this study were located on the same evolutionary branch (Figure 2A), suggesting a common origin.
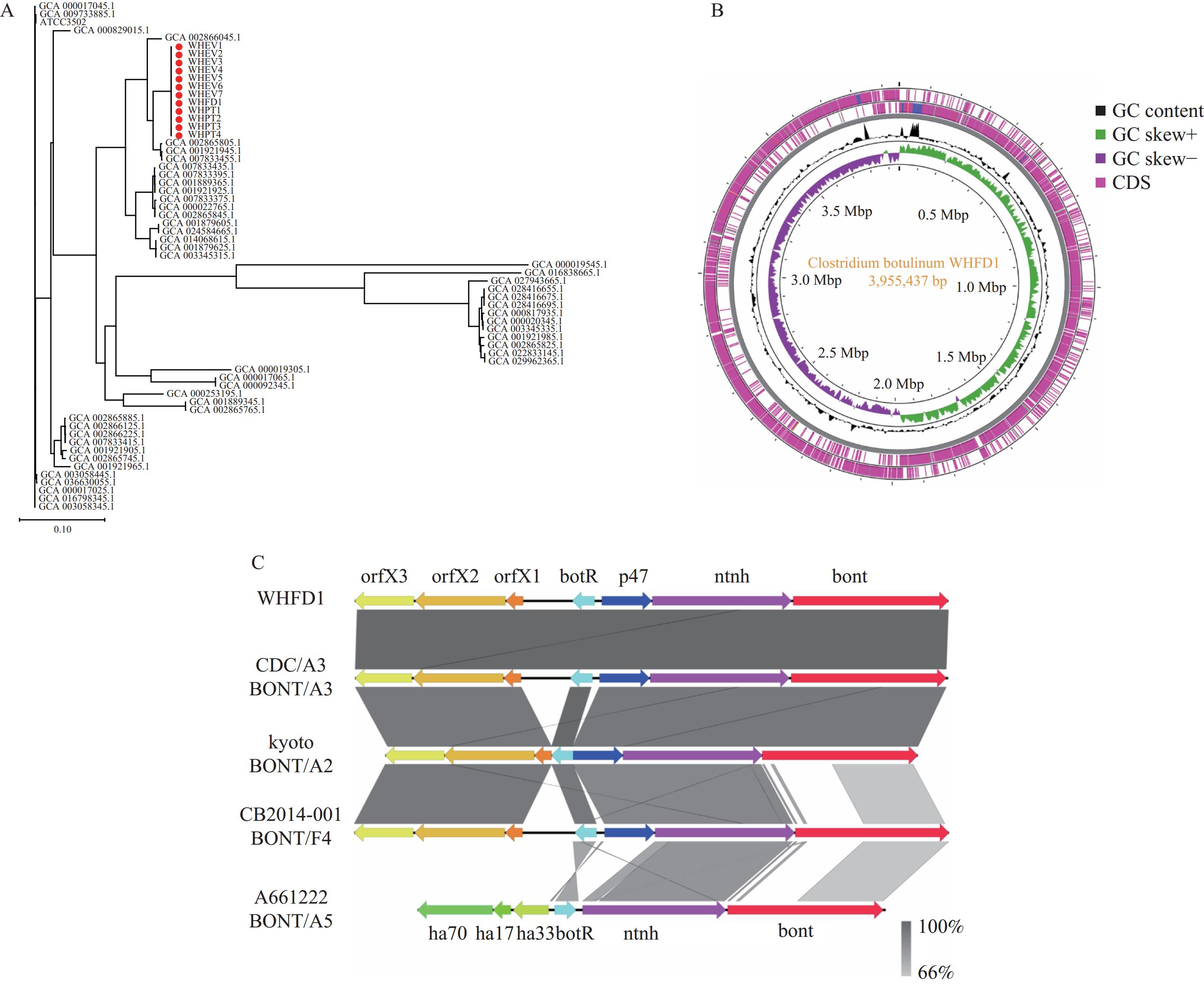 Figure 2.
Figure 2.The genomic characteristics of C. botulinum strains in this study. (A) Phylogenetic tree of C. botulinum strains based on coreSNP analysis; (B) Circular map of the WHFD1 isolate strain genome; (C) Comparison of the bont gene cluster arrangements in the WHFD1 genome and four C. botulinum genomes downloaded from GenBank.Note: The label names marked with red dots in panel A are the sequences of this study; These circles in panel B represent (from inside to outside): 1) GC skew, 2) GC content, 3) and 4) coding sequences (CDS); the different colors in panel C represent different genes in the bont gene clusters. The gradient of gray color represents the different BLAST identity values.
To investigate the genomic characteristics of C. botulinum in this study, we performed whole-genome sequencing (WGS) of the WHFD1 strain isolated from pickled eggs. Sequencing revealed that the WHFD1 genome comprised a circular chromosome and a circular plasmid (Figure 2B), with genome sizes of 3,955,437 bp and 243,977 bp, respectively. The GC contents were 28.19% and 25.48%, respectively. Annotation indicated that these genomes harbored 3,554 and 298 coding DNA sequences (CDS) regions, respectively. Average nucleotide identity (ANI) analysis showed that the chromosomal genome of WHFD1 shared 97.53% identity with the C. botulinum reference sequence ATCC3502, confirming the identification of WHFD1 as C. botulinum. The bont gene in the WHFD1 genome was located on the plasmid, with no bont gene detected on the chromosome. BLAST comparison showed that the bont gene had the highest homology with the bont/A3 subtype.
Furthermore, we analyzed the bont gene cluster encoding botulinum neurotoxin in the WHFD1 isolate. We extracted the bont gene cluster from the WHFD1 genome and downloaded four distinct bont gene clusters (A3, A2, F4, and A5) from the GenBank database. Using Easyfig (version 2.2.5, Brisbane, Australia) software (2), a collinearity analysis was performed, revealing that the bont gene clusters of the WHFD1 isolate shared the same arrangements and locations as BoNT/A3 CDC/A3 (Figure 2C). They both belong to the typical orfX+ gene cluster, which consists of the bont gene and five accessory genes. Although BoNT/A2 Kyoto and BoNT/F4 CB-2014001 also belong to the orfX gene cluster, their bont gene sizes and arrangements differ partially from that of the WHFD1 strain. The collinearity analysis confirmed that the WHFD1 strain belonged to the BoNT/A3 subtype, consistent with the BLAST result.
In this incident, five cases presented with similar symptoms, including blurred vision and ptosis as the primary symptoms, accompanied by difficulties swallowing, speaking, and breathing. These findings were consistent with the characteristics of botulinum toxin poisoning. After treatment with botulinum antitoxin, significant improvements were observed.
The qPCR assays conducted on fecal samples from the patient and pickled eggs for BoNT/A C. botulinum both yielded positive results. Twelve strains of C. botulinum were isolated from the pickled eggs, rectal swabs and feces of patients, and environmental specimens. Phylogenetic analysis confirmed that they originated from the same source. Whole-genome sequencing (WGS) revealed that all strains belonged to the BoNT/A3 subtype.
Based on a comprehensive analysis of the clinical symptoms, epidemiological investigation results, and laboratory test findings, we concluded that this outbreak was a case of foodborne botulism caused by homemade pickled eggs contaminated with C. botulinum producing BoNT/A3.
-
On July 19, the Weihai CDC published “Summer Alarm Bell: Foodborne Disease Crisis Caused by Botulinum Toxin” online, which provided a detailed introduction to botulinum toxin, poisoning symptoms, high-risk food sources, and preventive measures. The publication recommended avoiding homemade fermented and pickled foods.
Additionally, we suggest that medical institutions maintain a reserve supply of botulinum antitoxin for emergency use.
-
C. botulinum is a strictly anaerobic, Gram-positive bacterium whose spores are ubiquitous in soil and livestock feces. When foods such as fruits, vegetables, meat, and grains are contaminated by C. botulinum spores, the bacteria can proliferate rapidly and produce toxins under anaerobic conditions. In 1897, van Ermengem first isolated a strain of C. botulinum from salted ham associated with a foodborne botulism outbreak in Belgium (3). In China, Wu et al. first confirmed the presence of botulism in Xinjiang in 1958 (4). As of 2020, a total of 22 PLADs in China have reported cases of foodborne botulism (5). Most foodborne botulism cases occurred in northwestern China, including Xinjiang and Qinghai. The most common contaminated sources of botulism were homemade pickled foods, such as stinky tofu and dried beef (5).
In this study, the contaminated food source was pickled eggs. Epidemiological investigations revealed that the eggs originated from free-range hens within the village. Their surfaces may have been contaminated by C. botulinum from soil or livestock feces. Inadequate cleaning before pickling and the anaerobic conditions produced during the pickling process likely led to botulinum toxin production. Botulinum toxin is heat-labile and can be completely destroyed by heating at 80 ℃ for 30 minutes or 100 ℃ for 10 to 20 minutes. In this case, individuals consumed the pickled eggs without heating. We speculate that this was the primary cause of this foodborne botulism outbreak. We recommend thoroughly heating pickled foods before consumption to eliminate botulinum toxin and avoid similar incidents.
We used WGS to perform source tracing analysis, strain typing, and virulence gene subtyping. Core SNP analysis indicated that the 12 C. botulinum isolates originated from the same source. This finding was consistent with epidemiological investigation results and provided evidence for source tracing analysis. C. botulinum can be classified into seven serotypes (A to G) based on antigenic properties. Through WGS and BLAST comparison, all isolated strains were confirmed as BoNT/A3. The BoNT/A3 subtype is frequently found in the Southern Hemisphere, with outbreaks reported in countries such as Australia and Argentina (6). BoNT is encoded by the bont gene cluster, which is located on either the chromosome or plasmid of Clostridium species. The bont gene clusters are classified into two types: orfX+ and ha+. The bont/A3 gene belongs to the orfX+ gene cluster, which comprises the botulinum neurotoxin-encoding gene (bont) and several accessory genes, including orfX1, orfX2, orfX3, botR, p47, and ntnh (7). Obtaining the complete C. botulinum genomes in this study allowed us to identify the locations and arrangements of bont gene clusters within the isolates. This is important for strain typing and virulence gene detection.
This investigation revealed that foodborne botulism remains a public health issue in China. Therefore, strengthening education regarding the health risks of consuming homemade, traditionally pickled foods is critical to safeguarding public health.
-
The authors thank Shandong CDC and China CDC for the technical guidance during the survey.
HTML
| Citation: |



 Download:
Download:




