-
Co-infection with Mycobacterium tuberculosis (MTB) and human immunodeficiency virus (HIV) has emerged as a growing global concern. HIV-positive cases accounted for 6.3% of incident cases and 14.8% of deaths among all tuberculosis (TB) cases in 2022 (1). The World Health Organization (WHO) has recommended the inclusion of the C-reactive protein (CRP) test in the active tuberculosis (ATB) screening algorithm in its latest guidelines (2). CRP is a non-specific inflammatory biomarker that rises when the body encounters interleukin-6-induced pyogenic infection (such as TB). Research shows that the CRP test has similar sensitivity to, and better specificity than, WHO-recommended four-symptom screening (W4SS) among people living with HIV (PLHIV) in all sub-populations. With a negative predictive value of 97.3%, the CRP test is an ideal rule-out exam to reduce the number of individuals who need further confirming tests by 36%, thus saving a large amount of health resources (2-3). The performance of the CRP test in African regions has been well-documented (4). However, the suitability and effectiveness of the CRP test in low HIV burden and moderate TB burden regions, such as Shanghai Municipality, China, are currently unknown. This study utilized health service data from all health facilities in Shanghai, along with patient management data of infectious diseases, to assess the use of the CRP test and calculate the sensitivity and specificity of CRP results in relation to TB screening. Propensity score matching (PSM) was employed to match HIV-infected and HIV-uninfected tuberculosis patients. A receiver operating characteristic (ROC) curve was plotted, and the area under the curve (AUC), optimal sensitivity, and specificity were determined. Among included participants, 94 TB/HIV patients, 986 HIV-negative TB patients, and 282 PLHIV (excluding TB) were matched to at least one CRP test record. We simulated TB screening and plotted the ROC curve, with AUC values of 0.616 for the entire study population and 0.801 for PLHIV only. The best CRP threshold for PLHIV is 11.115 mg/L. Our study offers a promising opportunity to screen for tuberculosis among PLHIV using the CRP test in Shanghai.
In this study, patient information was collected for individuals with active pulmonary tuberculosis and PLHIV from the China Information System for Disease Control and Prevention (CISDCP). The ATB patients were diagnosed between January 1, 2018 and October 31, 2023, according to the National Industry Standard (WS 196-2017). Individuals with non-tuberculous mycobacteria and old tuberculosis lesions were excluded. PLHIV individuals were included if they were diagnosed and under surveillance before October 31, 2023. The CRP test results were extracted from the Survey System of Health Resources and Medical Services (SSHRMS) database that were associated with ATB and PLHIV individuals using a unique identification code. The test results were collected from 100 days before to 30 days after the date of diagnosis for ATB patients and from January 1, 2021 to October 31, 2023, for PLHIV. In cases where an individual had multiple CRP test results, the record closest to the date of TB treatment for ATB patients or the latest result for PLHIV was included in the analysis.
Microsoft Excel (version 2010, Microsoft Corporation, Redmond, United States) was used for data preprocessing, including converting character variables to numerical variables, data extraction, and grouping. R (version 4.3.1, R Foundation for Statistical Computing, Vienna, Austria) was used to conduct all statistical analyses. PSM was used to match HIV-infected and non-infected tuberculosis patients at a proportion of 1:10 to control for confounding factors such as gender and age. Propensity score is a mathematical method to reduce selection bias by balancing covariates between treatment and control groups. The caliper value of the PSM was set to 0.01. A rank sum test of multiple groups of independent nonparametric samples was performed, and the Baumgartner-Wei-Schindler test was used for pairwise comparisons between groups. A receiver operating characteristic (ROC) curve was plotted, and the area under curve (AUC), optimal sensitivity, and specificity were calculated. A P<0.05 was considered statistically significant in all analyses.
A total of 153 TB/HIV co-morbid patients were diagnosed between January 1, 2018 and October 31, 2023. These patients were matched with 1,530 non-HIV-infected TB patients using PSM, forming one group. Another group, serving as the control, consisted of 1,033 PLHIV individuals without ATB or TB history. Before PSM, the raw treatment and control groups differed substantially (Figures 1A and 1B). After PSM, however, homogeneity improved significantly among the study groups (Figures 1C and 1D).
 Figure 1.
Figure 1.Comparison of propensity scores between TB/HIV and HIV-negative TB patients before and after PSM in Shanghai Municipality, China, 2021–2023. (A) TB/HIV before PSM; (B) HIV-negative TB patients before PSM; (C) TB/HIV after PSM; (D) HIV-negative TB patients after PSM.
Abbreviation: TB=tuberculosis; HIV=human immunodeficiency virus; PSM=propensity score matching.A total of 3,560 test records were successfully extracted according to the extraction rules described above. After matching these records with the included population, 94 of 153 TB/HIV patients, 986 of 1,530 HIV-negative TB patients, and 282 of 1,033 PLHIV (excluding tuberculosis) were found to have at least one CRP test record (Table 1).
Group TGroupB/HIV, N=94 HIV-TB (after PSM), N=986 PLHIV (non-TB), N=282 Gender Male 90 908 261 Female 4 78 21 Age group (years) 20–29 13 94 47 30–39 18 198 109 40–49 23 235 43 50–59 20 238 37 60–69 15 163 34 70–79 5 58 12 Abbreviation: TB=tuberculosis; HIV=human immunodeficiency virus; PLHIV=people living with HIV; CRP=C-reactive protein; PSM=propensity score matching. Table 1. General characteristics of all participants with CRP results, Shanghai Municipality, China, 2021–2023.
CRP results were recoded based on actual test values using cutoff values of >5 mg/L and >10 mg/L. Results for different cutoff values are presented in Table 2.
Group TB/HIV HIV-TB (after PSM) PLHIV (non-TB) P CRP >5 mg/L Positive count (N, %) 76, 80.9 519, 52.6 117, 41.5 <0.001 Mean positive CRP 57.2 46.5 24.8 Negative count (N, %) 18, 19.2 467, 47.4 165, 58.5 Mean negative CRP 2.2 1.6 1.7 CRP >10 mg/L Positive count (N, %) 68, 72.3 420, 42.6 63, 22.3 <0.001 Mean positive CRP 63.0 55.7 39.4 Negative count (N, %) 26, 27.7 566, 57.4 219, 77.7 Mean negative CRP 3.9 2.6 3.2 Abbreviation: TB=tuberculosis; HIV=human immunodeficiency virus; PLHIV=people living with HIV; CRP=C-reactive protein; PSM=propensity score matching. Table 2. Count and mean of CRP results by >5 mg/L and >10 mg/L cut-off value, Shanghai Municipality, China, 2021–2023.
Using CRP results, we conducted TB screening among PLHIV and plotted the ROC curve (Figure 2). The AUC was 0.616 for the entire study population and 0.801 for PLHIV. The difference was statistically significant (P<0.001). Based on the ROC curve, the optimal CRP threshold for PLHIV was 11.115.
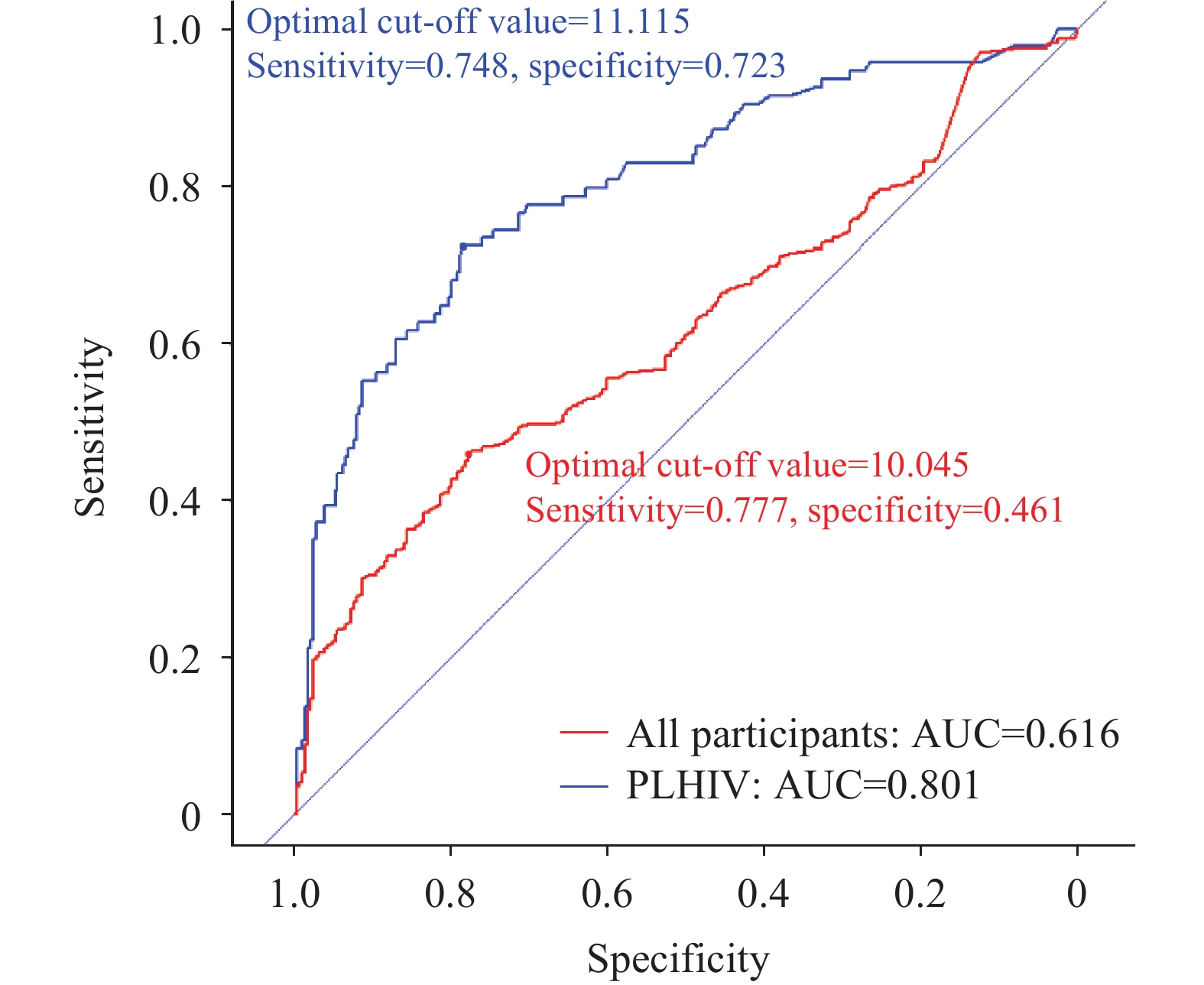 Figure 2.
Figure 2.ROC curve of CRP screening for TB in all participants and PLHIV only, Shanghai Municipality, China, 2021–2023.
Abbreviation: ROC=receiver operating characteristic; CRP=C-reactive protein; TB=tuberculosis; HIV=human immunodeficiency virus; PLHIV=people living with HIV.For PLHIV, at a CRP threshold of >5 mg/L, the sensitivity was 80.85% and the specificity was 58.51%. At a CRP threshold of >10 mg/L, the sensitivity was 72.23% and the specificity was 77.66%. For all participants, the sensitivity decreased to 55.09% (CRP>5 mg/L) and 45.19% (CRP>10 mg/L), while the specificity remained unchanged.
-
This study represents the first exploration of the feasibility and effectiveness of using CRP testing for TB screening among PLHIV in Shanghai. This study utilized resources from a big data platform, cross-matching data from the CISDCP. Because the proportion of TB/HIV co-infected individuals among all TB patients in Shanghai was relatively small, and significant differences in population characteristics were observed, PSM was employed to match individuals with similar characteristics from the entire TB patient population. Matching was based on significant TB/HIV population characteristics, such as gender and age, to minimize potential confounding factors between study groups. Following PSM, the demographic characteristics of the study population were fairly homogeneous.
After PSM, 153 patients had both TB and HIV, 1,530 patients had only TB, and 1,033 patients had only HIV. However, the proportion of cases with matching CRP results from the big data platform was low: 64.17% (1,080/1,683) among active tuberculosis patients and only 31.70% (376/1,186) among HIV-positive patients. This indicates that CRP testing is not routinely used in the diagnosis and treatment of tuberculosis patients or in the follow-up examinations of HIV patients. Furthermore, our study found that of the 3,560 CRP results extracted, only 12 used POCT, accounting for a mere 0.3% of the total results. Although the WHO recommends the use of POCT-based CRP tests as a screening tool for TB in PLHIV, our findings indicate that its application is quite limited. The practical difficulties are that, in current medical facilities in Shanghai, CRP tests typically require whole blood or serum samples, professional technicians, laboratory equipment, and higher costs, restricting its use as a screening tool.
We collected original CRP results from medical facilities and classified them by cutoff values of 5 mg/L and 10 mg/L, following WHO recommendations, to compare CRP level differences among TB/HIV patients, HIV-negative TB patients, and HIV-positive individuals without ATB. Both the positive rate and mean CRP result in TB/HIV patients were the highest, with significant differences compared to the other two groups under both cutoff values. We also simulated TB screening using CRP and drew the ROC curve. CRP’s effect was significantly better in PLHIV than in the general population, indicating that CRP testing is more suitable for TB screening in PLHIV. This is consistent with Meyer et al.’s conclusion (5). According to our data, the best CRP cutoff value for PLHIV is 11.115, with 72.23% sensitivity and 77.66% specificity. Lowering the cutoff to the WHO-recommended 5 mg/L for TB screening increases sensitivity to 80.85% but decreases specificity to 58.51%. Our results are similar to studies in India (optimal cutoff: 8.25 mg/L, sensitivity: 70.13%, specificity: 69.86) (6), Durban (cutoff: >10 mg/L, sensitivity: 78.6%, specificity: 72.3%) (7), and Kampala (cutoff: ≥10 mg/L, sensitivity: 73%, specificity: 80%) (8). Some studies show better results (4,9–10), with sensitivity reaching the WHO standard for an ideal screening tool (90%), but almost none meet the specificity standard (70%).
Finally, we must emphasize that our data were collected based on existing medical behaviors that probably were not originally intended for TB screening. Also, as a nonspecific index, CRP levels could be elevated by other concomitant infections or acute responses. Therefore, our results can only be considered an indication of the potential use of CRP as a TB screening tool among PLHIV, rather than a quantitative measure of CRP levels. This represents a significant limitation of our study, which warrants further research.
In conclusion, this study provides a promising approach to screening for TB in PLHIV using a simple blood index in this city with a population of over 30 million permanent residents. Any public health decision, no matter how small, requires significant societal resources multiplied by this population size. This study offers an original standpoint for future research to explore the method, process, algorithm, optimal screening threshold, health economic evaluation, and other details.
HTML
| Citation: |



 Download:
Download:




