-
The global challenge of tuberculosis (TB) recurrence after successful treatment persists despite national TB programs primarily focusing on patient detection and management of treatment. These programs often overlook the need for follow-up and intervention post-treatment. Although the incidence of TB in China has been steadily declining, significant efforts are still required to achieve the END-TB targets. This study utilizes a transmission dynamic model to evaluate the impact of various control strategies on accelerating TB elimination in China. Under the current strategies, TB incidence rates in China are projected to decrease to 44.9 per 100,000 by 2030 and to 40.4 per 100,000 by 2035. Introducing post-recovery interventions to prevent recurrence, along with providing TB preventive treatment (TPT) to 30% of individuals with latent TB infection (LTBI) starting in 2025 and increasing LTBI TPT coverage to 90% beginning in 2031, will contribute to achieving the milestones of the End TB Strategy by 2030 and the 2035 target.
TB continues to pose a significant threat to public health worldwide, including in China. The World Health Organization’s (WHO) End TB strategy targets a reduction in global TB incidence by 80% by 2030 and by 90% by 2035 relative to 2015 levels. In 2022, China reported an estimated 748,000 TB cases, ranking third globally with an incidence rate of 55 cases per 100,000 people. From 2000 to 2022, the annual average percentage change (AAPC) in TB incidence in China was approximately 3.1%, surpassing the global rate of 1.2% during the same timeframe (1). However, this progress remains substantially below the WHO’s target AAPC of 10% by 2025, necessary to meet the 2030 milestone of the End TB Strategy. Although most TB patients can be effectively treated with chemical therapy, the recurrence of TB post-treatment presents a significant hurdle. In 2022, there were over 350,000 cases of recurrent TB globally, constituting 5.5% of the 6.43 million newly reported cases. This issue is particularly acute in high-burden countries such as the Russian Federation, where the rate reaches 21%. Recurrent TB often involves more complicated pathologies, increasing the risk of clinical complications and drug resistance, thereby complicating treatment and management, and exacerbating transmission risks. This study aims to develop a dynamic model to analyze TB incidence in China from 2005 to 2022 and forecast trends up to 2030 and 2035, considering both existing strategies and hypothetical scenarios with enhanced measures for recurrence control.
Recurrence was defined as the condition in which patients, previously treated and declared cured or having completed treatment for TB, are diagnosed with a subsequent episode of TB, which could either be a true relapse or a new episode caused by reinfection (2). TPT was defined as the administration of treatment to individuals considered at risk for developing TB disease, with the aim of reducing this risk. This is also known as LTBI treatment or preventive therapy. The WHO recommends TPT for specific target populations, including people living with human immunodeficiency virus (HIV), household contacts of bacteriologically confirmed pulmonary TB patients, and others at heightened risk of TB such as individuals initiating antitumor necrosis factor (anti-TNF) treatment, those receiving dialysis, preparing for organ or hematological transplants, or having silicosis. Secondary preventive treatment was defined distinctly from TPT for LTBI, aimed to reduce the risk of recurrence following a successfully treated TB episode.
The compartmental model is a classical mathematical framework utilized to model the dynamics of infectious diseases. It categorizes individuals into distinct groups, with each group sharing average characteristics and typically exhibiting uniform interactions. In this study, a SEIR compartmental model was constructed to analyze the transmission dynamics of Mycobacterium tuberculosis (M. tb), incorporating the risk of recurrence based on a literature review (3-4). The simulations were conducted using the Epimodel package in R software (version 4.0.3; R Core Team, Vienna, Austria), and the model’s structure is depicted in Figure 1.
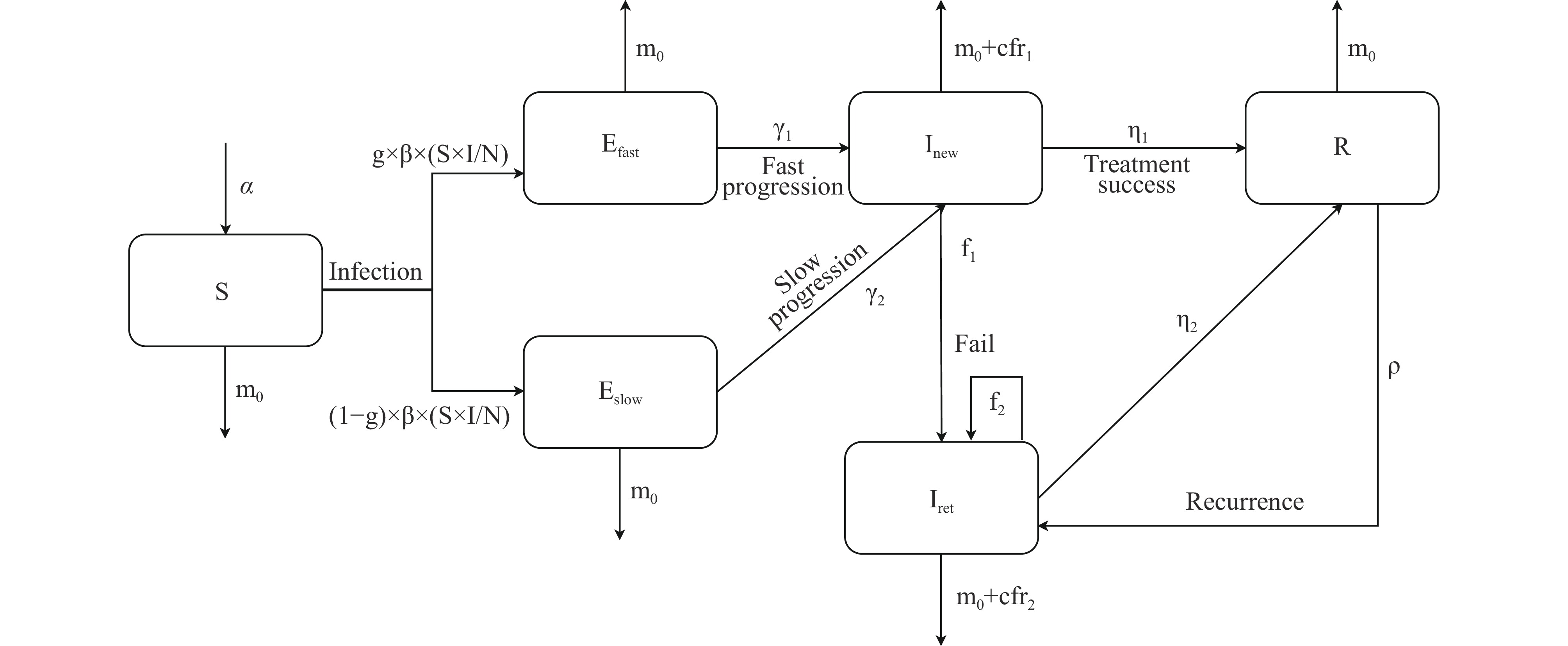 Figure 1.
Figure 1.The flow diagram of TB transmission dynamic model considering recurrence.
Note: N refers to the total population. α is the birth rate; β is the transmission rate of infectious cases; g is the proportion of slow progression; γ is the progressive rate (divided into γ1 and γ2 for fast and slow progression) from latent infection to infectious; η is the proportion of successful treatment (divided into η1 and η2 based on treatment history); f is the proportion of treatment failure (divided into f1 and f2 based on treatment history); m0 is the natural mortality rate; cfr is the fatality rate of infectious due to TB disease (divided into cfr1 and cfr2 based on treatment history); ρ is the recurrence rate from recovered individuals.
Abbreviation: TB=tuberculosis.
The model delineates several states in the progression of TB. Susceptible (S): Individuals who have not been infected with M. tb. Latent Infection (E): Individuals who are infected with M. tb but have not developed active disease are subdivided into fast-progressing (Efast) and slow-progressing (Eslow) categories. Previous research indicates that the lifetime risk of progressing to active TB post-infection ranges from 5%–10%, with approximately 50% developing the disease within the first 2–5 years (γ1) and the others during subsequent periods (γ2). Infectious (I): Active TB cases, split into newly diagnosed, untreated patients (In) and those with previously treated TB (Ir). Recovered (R): Individuals who have either been cured or completed their treatment regimen. The model outlines potential outcomes for TB patients: ① cure or completion of treatment (η1, η2), ② treatment failure (f1, f2), and ③ death attributed to TB (m1, m2) or other causes (m0). Additionally, individuals in the recovered category (R) may suffer a recurrence (ρ), transitioning back to being infectious (Ir).
The equations of the model are as follows:
$$\begin{aligned} & \mathrm{N(0)=S(0)+E(0)+I(0)+R}(0)\\& \mathrm{m}_{ \mathrm{0}} \mathrm{+cfr+\eta +f=1}\\& \mathrm{f}_{ \mathrm{1}} \mathrm{=1-m}_{ \mathrm{0}} \mathrm{-cfr}_{ \mathrm{1}} \mathrm{-\eta }_{ \mathrm{1}} \\ & \mathrm{f}_{ \mathrm{2}} \mathrm{=1-m}_{ \mathrm{0}} \mathrm{-cfr}_{ \mathrm{2}} \mathrm{-\eta }_{ \mathrm{2}} \\ & \mathrm{\Delta S=\alpha -\beta \times (S \times I/N)-m}_{ \mathrm{0}} \mathrm{ \times S} \\& \mathrm{\Delta E}_{ \mathrm{fast}} \mathrm{=(1-g) \times \beta \times (S \times I/N)-\gamma }_{ \mathrm{1}} \mathrm{ \times E}_{ \mathrm{fast}} \mathrm{-m}_{ \mathrm{0}} \mathrm{ \times E}_{ \mathrm{fast}} \\& \mathrm{\Delta E}_{ \mathrm{slow}} \mathrm{=g \times \beta \times (S \times I/N)-\gamma }_{ \mathrm{2}} \mathrm{ \times E}_{ \mathrm{slow}} -\mathrm{m}_{ \mathrm{0}} \mathrm{ \times E}_{ \mathrm{slow}} \\& \mathrm{\Delta I}_{ \mathrm{n}} \mathrm{=\gamma }_{ \mathrm{1}} \mathrm{ \times E}_{ \mathrm{fast}} \mathrm{+\gamma }_{ \mathrm{2}} \mathrm{ \times E}_{ \mathrm{slow}} \mathrm{-I}_{ \mathrm{n}} \\& \mathrm{\Delta I}_{ \mathrm{r}} \mathrm{=f}_{ \mathrm{1}} \mathrm{ \times I}_{ \mathrm{n}} \mathrm{+f}_{ \mathrm{2}} \mathrm{ \times I}_{ \mathrm{r}} \mathrm{-\eta }_{ \mathrm{2}} \mathrm{ \times I}_{ \mathrm{r}} \mathrm{-(m}_{ \mathrm{0}} \mathrm{+cfr}_{ \mathrm{2}} \mathrm{) \times I}_{ \mathrm{r}} \mathrm{+\rho \times R}\\& \mathrm{\Delta R=\eta }_{ \mathrm{1}} \mathrm{ \times I}_{ \mathrm{n}} \mathrm{+\eta }_{ \mathrm{2}} \mathrm{ \times I}_{ \mathrm{r}} \mathrm{-\rho \times R-m}_{ \mathrm{0}} \mathrm{ \times R} \end{aligned} $$ The parameters β, γ1, and γ2, along with the initial values for the compartments Efast(0) and Eslow(0), were calibrated in the model using the optim() function, utilizing TB incidence data in China from 2013 to 2022. The aim was to minimize the root mean square error (RMSE) between the predicted and observed data. Initial values for Inew(0), Iret(0), and R(0) were derived using data from the Chinese national TB surveillance system, underreporting survey, and the WHO country database. Demographic data were sourced from the China Statistical Yearbook 2023, while treatment-related parameters were computed from the national TB surveillance system. Natural history parameters were drawn from prior research (Table 1).
Parameter/compartment Definition Estimated value Resource $ \text{α} $ Birth rate Annual birth rates during 2005–2022 (‰) China Statistical Yearbook (5) $ {m}_{0} $ Natural mortality rate Annual natural mortality rates during 2005–2022 (‰) China Statistical Yearbook (5) $ g $ Proportion of individuals with slow progression among latent infections 91% Ragonnet et al. (3) $ {\eta }_{1} $ Successful treatment rate for new patients 94% National TB surveillance system $ {\eta }_{2} $ Successful treatment rate for retreated patients 85% National TB surveillance system $ {cfr}_{1} $ Case fatality rate for new patients 3% Straetemans et al. (6) $ {cfr}_{1} $ Case fatality rate for retreated patients 9% Straetemans et al. (6);
Mathew et al. (7)$ \beta $ Transmission rate 2.35 Model fitting $ {\gamma }_{1} $ Progressive rate for slow progression LTBI 0.038 Model fitting $ {\gamma }_{2} $ Progressive rate for fast progression LTBI 0.00046 Model fitting $ \rho $ Recurrence rate for recovered patients 0.0005 National TB surveillance system $ N\left(0\right) $ General population in 2013 1,367,260,000 China Statistical Yearbook (5) $ S\left(0\right) $ Susceptible patients in 2013 1,104,396,330 N(0)-E(0)-I(0)-R(0) $ E\left(0\right) $ LTBI in 2013 247,200,608 Gao et al. (8) $ {E}_{fast}\left(0\right) $ Fast progression among LTBI in 2013 20,996,170 Model fitting $ {E}_{slow}\left(0\right) $ Slow progression among LTBI in 2013 226,204,438 Model fitting $ {I}_{new}\left(0\right) $ Newly diagnosed TB patients 884,206 Chinese national TB surveillance system/ underreporting survey/ WHO country database $ {I}_{ret}\left(0\right) $ Previously treated TB patients 81,097 Chinese national TB surveillance system/ underreporting survey/ WHO country database $ R\left(0\right) $ Recovered 14,558,870 Chinese national TB surveillance system/ underreporting survey/ WHO country database Abbreviation: WHO=World Health Organization; TB=tuberculosis; LTBI=latent tuberculosis infection. Table 1. Estimated values of parameters and initial compartments.
Six scenarios, including one baseline and five intervention scenarios to be initiated from 2025, were designed to model current and potential future TB incidence in China. Baseline Scenario: reflects current conditions without additional interventions. Scenario 2 (Recurrence-Free Treatment Scenario): projects a 90% decrease in recurrence rate through novel treatment regimens. Scenario 3 (solely Post-Treatment Intervention Scenario): incorporates an 85% reduction in recurrence risk, deploying new vaccines and secondary preventative treatments, along with follow-up for recovered TB patients. Scenario 4 (Scenario 3 + TPT for 30% LTBI): expands on Scenario 3 by including TPT for 30% of the LTBI population starting in 2025 to expedite progress toward the 2030 End TB strategy milestone. The efficacy of TPT in reducing TB risk among the LTBI population is estimated at 90%. Scenario 5 (Control against Scenario 4): focuses on the LTBI intervention alone as a comparison to Scenario 4 with an identical outcome, increasing TPT coverage to 50% of the LTBI population. Scenario 6 (Scenario 4 + Enhanced TPT for 90% of the LTBI Population Beginning in 2031): Extends Scenario 4 by raising the TPT coverage for the LTBI population to 90% starting in 2031, accelerating achievement of the 2035 End TB strategy goals.
-
Under the baseline scenario, it is anticipated that the incidence of TB in China will decrease to 44.9 per 100,000 by 2030 and further to 40.4 per 100,000 by 2035. Should a new “recurrence-free” regimen be introduced from 2025 (Scenario 2), the incidence is expected to drop to 43.6 per 100,000 by 2030, indicating a 2.9% additional reduction compared to the baseline. Implementing post-recovery interventions for all recovered TB patients (Scenario 3) could lead to an incidence rate of 38.3 per 100,000 by 2030, marking a 14.7% greater decline relative to the baseline. Introducing TPT for 30% of LTBI cases in addition to Scenario 3 measures (Scenario 4) could further lower the incidence to 29.2 per 100,000 by 2030, thus meeting the 2030 End TB Strategy milestone of less than 30 per 100,000 with a 35.0% greater reduction compared to the baseline. Implementing TPT alone for 50% of LTBI cases (Scenario 5) could reduce the incidence to 28.8 per 100,000 by 2030. Scenario 4 would require a total of 98.5 million recovered patients and LTBI cases to receive preventive treatment, while Scenario 5 would entail treatment for 123.8 million LTBI cases. Scenario 4 could result in cost savings of 4.6 to 7.9 billion Chinese Yuan (CNY) over Scenario 5 by reducing the number of treatment recipients by 25.4 million, assuming treatment costs between 180–310 CNY per person for a 6-month course of isoniazid (6H) or a 3-month regimen of isoniazid combined with rifampentine (3HP). Extending the coverage of LTBI preventive treatment to 90% beyond 2030 as per Scenario 4 could reduce the incidence to 9.7 per 100,000 by 2035, thereby achieving the 2035 End TB Strategy goal of less than 10 per 100,000 (Figure 2).
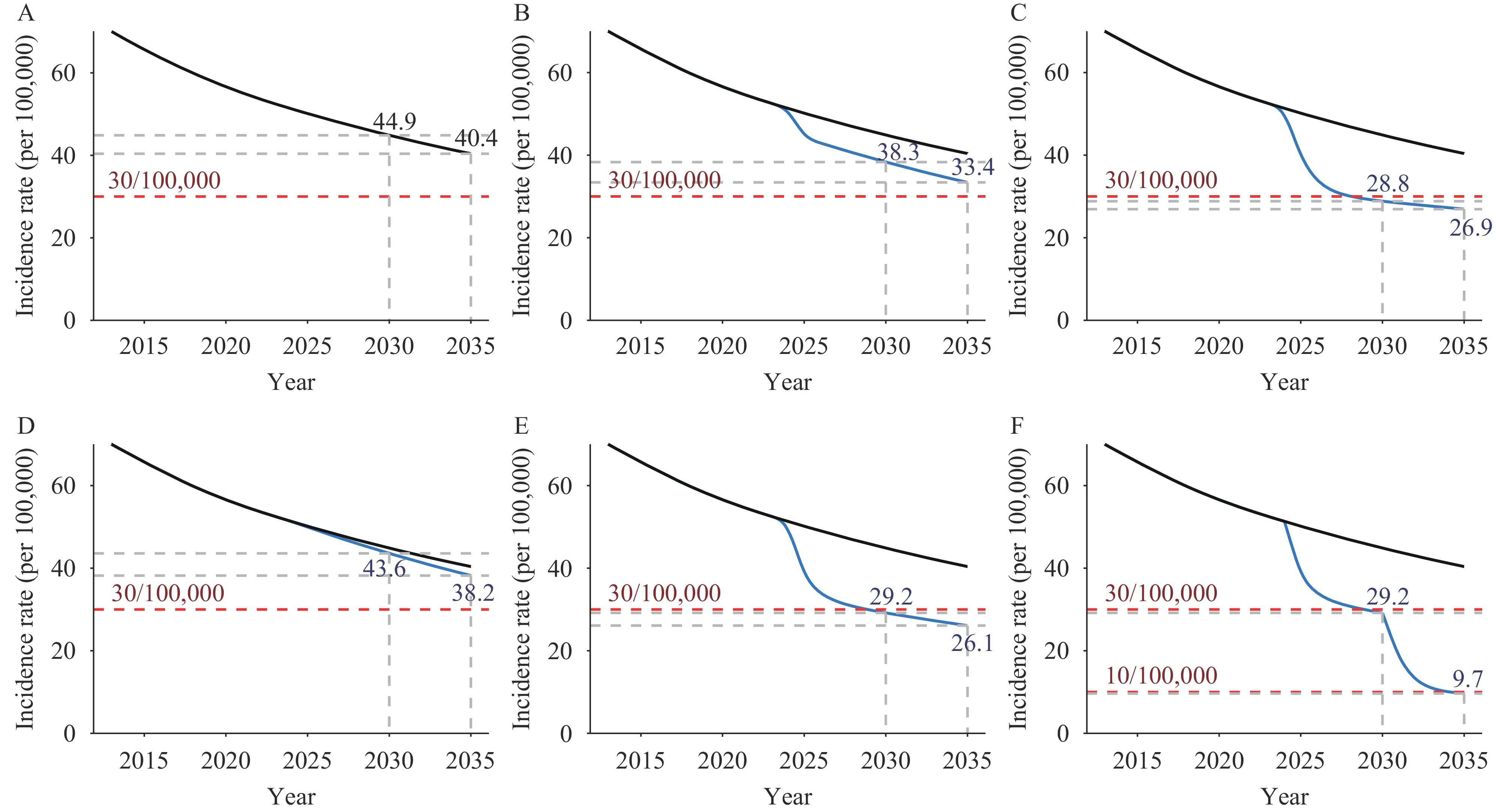 Figure 2.
Figure 2.Predictions of TB incidence in China with different interventions, 2013–2035. (A) Baseline scenario; (B) Scenario 2 − Recurrence free treatment scenario; (C) Scenario 3 − Sole Post − Treatment Intervention Scenario; (D) Scenario 4 − Scenario 3 + TPT for 30% LTBI; (E) Scenario 5 − Control scenario against scenario 4; (F) Scenario 6 − Scenario 4 + TPT for 90% LTBl since 2031.
Abbreviation: TB=tuberculosis; TPT=tuberculosis preventive treatment; LTBI=latent tuberculosis infection. -
The TB incidence rate in China showed a continuous downward trend. However, it is difficult to achieve the global End TB Strategy milestone and target with the current strategies. Implementing post-recovery interventions combined with 30% LTBI TPT from 2025 and expanding LTBI TPT coverage to 90% from 2031 will help achieve these goals.
Patients with TB exhibit a recurrence risk significantly higher than the incidence risk in the general population. Studies indicate that the recurrence rate in Shanghai Municipality is more than 18 times the local incidence rate. Reducing recurrence is crucial for assessing the clinical outcomes of novel TB vaccines and treatments. Recent clinical trials have tested several vaccines, and studies on treatment regimens that incorporate new second-line anti-TB drugs, such as Bedaquiline, Delamanid, and Pretomanid, have demonstrated reduced recurrence rates in rifampin-resistant patients. Moreover, nursing interventions, including psychological support, health education, and dietary guidance, have been effective in improving treatment adherence and potentially reducing recurrence rates. However, enhancing treatment regimens, whether through new drugs, improved patient care, or combination with therapeutic vaccines, falls short of achieving the milestones set for the 2030 End TB Strategy (Scenario 2). These strategies primarily benefit newly diagnosed patients and fail to address the needs of a large number of previously treated patients who remain at high risk for recurrence.
Following up with TB patients post successful treatment is crucial for early detection of recurrence and for mitigating risk. Secondary preventive treatment with isoniazid has been shown to effectively reduce TB recurrence (9). Previous modeling studies have demonstrated that screening for LTBI and administering preventive treatment can rapidly decrease TB incidence (10). However, widespread screening in the general population is hampered by high costs and low acceptance of the TPT, particularly impeding global TPT progress, notably in China. Acceptance of secondary preventive treatment may be higher among previously treated TB patients due to their familiarity with the disease. This modeling study indicates that strategies to prevent recurrence in all previously treated TB patients — through routine follow-up, secondary preventive treatment, and potential re-vaccination — could significantly hasten the reduction of TB incidence. Despite these interventions, the 2030 milestone (Scenario 3) remains unachievable. When these strategies are accompanied by TPT for 30% of individuals with LTBI (Scenario 4), achieving the 2030 milestone is feasible. By contrast, without recurrence intervention, TPT would need to extend to 50% of infected individuals (Scenario 5) to accomplish the same outcome, significantly raising both costs and implementation challenges. Although Scenario 4 can meet the 2030 End TB milestone, it falls short of the 2035 target. Only by expanding TPT to 90% of individuals with LTBI (Scenario 6), a largely impractical goal, can the 2035 target potentially be met.
The study was subject to some limitations. First, the underlying causes of TB recurrence may include endogenous reactivation or exogenous reinfection, and the effectiveness of various recurrence control interventions may differ across settings with varying TB burdens. Additionally, other effective interventions, such as active case finding, can also accelerate the reduction in TB incidence rates. Future efforts to implement recurrence interventions should be tailored to specific local conditions. Second, the efficacy of secondary preventive treatment with isoniazid has only been confirmed in HIV-positive TB patients. We hypothesize that it is equally effective in HIV-negative TB patients, similar to those without a treatment history, but this assumption requires further validation in future studies. Finally, our model did not distinguish between different types of TB (pulmonary or extrapulmonary). Nevertheless, the transmission rates, which are critical for influencing the outcomes, were derived through model fitting. Moreover, the parameters sourced from the surveillance system encompassed data from all TB forms. Hence, our results inherently represent a blended effect of both pulmonary and extrapulmonary TB.
According to estimates by Dodd et al. (11), there were approximately 155 million TB survivors worldwide in 2020. Recurrence presents a significant obstacle to achieving the global objective of TB elimination. National TB programs predominantly emphasize patient detection and treatment management, while often neglecting post-treatment follow-up and interventions. Implementing strategies to reduce recurrence is critical for improving TB control efforts both in China and around the world. Additionally, the development of new drugs and vaccines should focus on preventing recurrence and expedite the assessment of these innovations.
HTML
| Citation: |



 Download:
Download:





