-
Ongoing mpox infections reported in countries outside of Africa exhibit distinct characteristics. These infections are primarily observed in men who have sex with men (MSM) (1). Sexual intercourse is responsible for transmitting 95% of these infections, with a prevalence of 98% among MSM (1). The majority of cases present with well-defined, firm skin lesions that display a central indentation. These lesions are commonly found on the genitalia, anogenital area, oral mucosa, or rectal mucosa, which aligns with sites of skin contact during sexual activities. Published studies indicate that up to 50% of these infections involve co-infection with at least one other sexually transmitted infection (STI) (2). Previous research has shown that 28%–47% of the confirmed cases involve individuals living with human immunodeficiency virus (HIV) (3).
The high rates of sexually transmitted infections (STIs) among MSM with mpox emphasize the importance of STI testing. Inflammatory and ulcerative mucosal conditions, as well as STI pathogens, are closely linked to the acquisition and transmission of HIV. In this report, we present a case of acute HIV infection in a person who was being evaluated for mpox at our hospital, a referral center for monkeypox virus (MPXV) infection in Beijing Municipality, China.
HTML
-
A 29-year-old cisgender man presented with a fever (maximum temperature of 39 °C) that began within the past 7 days after a night of heavy drinking following his layoff from work. He subsequently developed a sore throat, non-productive cough, vomiting, painful inguinal lymph node swelling, and skin lesions on his genitalia and perineum, as well as more distant lesions on his trunk, face, and limbs. On August 17, 2023, he was admitted to Beijing Youan Hospital as a suspected mpox case. He denied any relevant medical history and had no history of recent travel to areas with known infections or direct contact with individuals suspected or confirmed mpox before symptom onset. Physical examination revealed pharyngeal congestion, swollen tonsils, redness and swelling of the penis, and painful inguinal lymph node swelling, as well as diffuse skin lesions on the genitalia (Figure 1), trunk, face, and limbs.
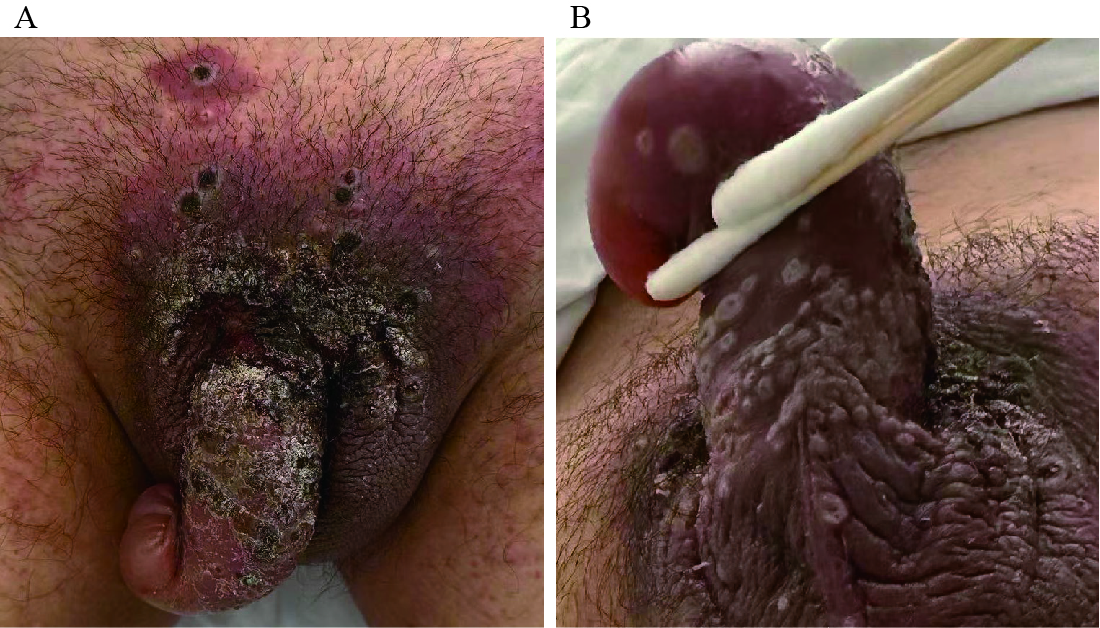 Figure 1.
Figure 1.Skin lesions on the genitalia and perineum of the confirmed mpox case. (A) Several grouped umbilicated whitish papules on the perineum; (B) several grouped umbilicated whitish papules on the genitalia.
He engaged in insertive condomless anal intercourse on August 3, 2023, with a partner whose HIV status was unknown. This occurred 14 days before the first appearance of a genital lesion. The individual reported conducting monthly HIV self-tests using rapid tests, as a precautionary measure due to concerns about acquired immunodeficiency syndrome (AIDS). All previous showed negative including the most recent one performed on August 10, 2023. The individual did not receive preexposure prophylaxis or postexposure prophylaxis for HIV.
Samples of skin lesions and oropharyngeal swabs were collected from the patient for MPX-specific real-time polymerase chain reaction (rtPCR) assays conducted at China CDC. Confirmed cases are defined as individuals with positive PCR assay results [cycle threshold (Ct) value <40] for any type of sample. The patient’s skin lesion (Ct 21.23) and oropharyngeal (Ct 31.97) samples tested positive for MPX PCR. The patient received care in an adult isolation unit where measures were taken to manage pain, prevent dehydration, and administer antibiotics for skin and soft tissue infection, according to National Guideline for Mpox Prevention and Control (4). As part of the routine initial laboratory assessment, we conducted an HIV rapid test using the HIV Ab test (Abogen Biosciences, China), which yielded a negative result. However, the patient’s CD4 cell count was found to be only 34 cells/mm3.
To further investigate, we performed a nonreactive 3rd generation serological test for HIV (Genscreen HIV 1/2, Bio-Rad). Subsequently, an HIV RNA test (m2000sp-m2000rt, Abbott Diagnostic, Chicago, IL) was conducted and showed a viral load of more than 1000,0000 copies/mL, indicating acute HIV infection. To verify these results, the HIV RNA test was repeated, yielding the same outcome.
On September 12, 2023, which was 39 days after the high-risk sexual behavior, an indeterminate result was obtained from the 4th-generation serological test for HIV (ARCHITECT HIV Ag/Ab Combo Abbott), along with the presence of p24 bands in the GEENIUS HIV 1/2 Bio-Rad immunoblot test (the Western Blot test, WB). Subsequently, on September 27, 2023, the 4th-generation serological test for HIV showed a reactive result, and on October 12, 2023, which was 69 days post the high-risk sexual behavior, the immunoblot test confirmed the presence of p17, p24, gp41, gp120, and gp160 bands. The results of the HIV tests conducted are summarized in Table 1. Following the diagnosis of acute HIV infection, the patient was initiated on antiretroviral treatment (ART) with bictegravir sodium, emtricitabine, and tenofovir alafenamide fumarate tablets on September 1, 2023.
Test items August 17, 2023 August 24, 2023 September 1, 2023 September 12, 2023 September 27, 2023 October
12, 2023November
5, 2023The viral shedding of MPXV
(Ct value)Skin lesion 21.23 NA NA NA Negative (scab) NA NA Oropharyngeal 31.97 NA NA 31 Negative NA Negative CD4 count (cells/mm3) 34 44 99 265 347 NA 410 HIV RNA (copies/mL) NA >10,000,000 >10,000,000 1,097 44 NA TND 3rd generation serology for HIV Negative Negative NA Negative Negative Positive Positive 4th generation serology for HIV NA NA NA Indeterminate Positive NA Positive Western blot NA NA NA Indeterminate NA Positive Positive (P24) (p17, p24, gp41, gp120, gp160) (p17, p24, p31, gp41, gp120, gp160) Note: The case reported a potential expose event of engaging in insertive condomless anal intercourse on August 3, 2023; and self-tested for HIV by a rapid HIV test with a negative result on August 10, 2023.
Abbreviation: NA=not available; TND=target not detected; MPXV=monkeypox virus; HIV=human immunodeficiency virus.Table 1. CD4 count, viral markers, and antibodies of HIV in the blood of the confirmed mpox case.
The patient was found to be negative for hepatitis B, hepatitis C, and syphilis. Table 2 displays the laboratory test results at admission and during the follow-up after medical evaluation. The patient did not experience any complications related to the disease. The symptoms improved rapidly, and the skin lesions were completely scabbed or desquamated. He was discharged on September 11, 30 days after the first lesion was reported. During a follow-up visit on November 5, 2023, approximately two months after initiating ART, the patient tested negative for HIV RNA, and his CD4 cell count was measured at 410 cells/mm3. The immunoblot test revealed the presence of p17, p24, p31, gp41, gp120, and gp160 bands, indicating a positive result. Additionally, a reactive 3rd-generation serological test confirmed the presence of HIV antibodies. The time from the history of HIV exposure within three months to the appearance of HIV antibodies was estimated.
Clinical index August 17, 2023 August 21, 2023 August 24, 2023 August 29, 2023 September 11, 2023 Total leukocytes count (×109/L) 11.80 4.41 1.80 1.95 5.24 Neutrophil count (×109/L) 8.30 3.49 1.20 1.36 3.50 Lymphocyte count (×109/L) 1.50 0.70 0.55 0.44 1.00 Monocyte count (×109/L) 1.80 0.20 0.05 0.07 0.70 Hemoglobin (g/L) 135 132 131 123 127 Platelets (×109/L) 157 155 152 177 439 C-reactive protein (mg/L) 156.0 75.0 19.1 24.5 18.1 Procalcitonin (ng/dL) 0.22 0.20 0.13 0.10 0.07 ALT (U/L) 32 30 23 25 31 AST (U/L) 35 36 42 41 40 Total bilirubin (μmol/L) 6.1 6.0 5.1 5.5 6.5 Albumin (g/L) 33.5 34.0 36.2 38.0 41.2 Creatinine (μmol/L) 85 83 81 82 88 Abbreviation: ALT=alanine transaminase; AST=aspartate aminotransferase. Table 2. Laboratory tests at admission and follow-up of medical assessment.
-
The confirmed mpox case with acute HIV coinfection was confirmed by a nonreactive HIV rapid test enzyme immunoassay (EIA) that detects IgG to HIV, a 3rd-generation EIA test (IgM sensitive) for HIV, and a positive HIV RNA test. Additionally, the results of the 4th-generation antigen-antibody combination EIA, which detects p24 antigen, HIV IgM, and IgG antibodies, changed from indeterminate to positive. The immunoblot test for the presence of p24 also changed from indeterminate to positive, confirming the patient’s acute HIV infection stage as Fiebig Ⅱ(5). The diagnosis of acute HIV is nearly always missed; however, most infected individuals remain ignorant of their HIV status until after the onset of AIDS. This is the first reported case of concurrent acute viral HIV and mpox coinfection in the Chinese mainland, highlighting the potential of mucosal surfaces as a route of concomitant acquisition of both infections. Similar cases of mpox with a concurrent acute HIV infection have been reported in Latin America, Portugal, and Brazil, where the HIV test results showed positive HIV RNA and positive HIV-1 antigen with a negative anti-HIV 1/2 antibody result (6–8). In an Italian case, mpox and recent HIV infection were diagnosed, and the patient had positive anti-HIV serology (9).
To our knowledge, the 3rd/4th generation HIV assays have shown higher sensitivity compared to the WB assay. In this case, both the 4th generation HIV serology assay and the WB assay yielded indeterminate results on September 12. However, the 3rd generation HIV assay using a gold-labeled reagent yielded a negative result. This discrepancy can be attributed to the different coated antigens used in the assays. The WB assay utilizes cleaved HIV-1 virus as the coated antigen, while the 3rd generation Abbott reagent uses genetically engineered gp41 and p24 antigens. The variations in the source and preparation of the coated antigens could explain why the window period for detecting single antibody reactions with the WB assay may be shorter than that of the 3rd generation gold-labeled reagent. Conversely, the window period for detecting multiple antibody reactions simultaneously with the WB assay may be longer than that of the 3rd generation gold-labeled reagent. This may account for the lack of an advantage of the 3rd generation HIV assay over the WB assay in this case.
On October 12th, both the 3rd generation HIV serology assay and the WB assay for multiple antibodies yielded positive results. This case presents an instance of early-stage acute HIV infection, characterized by the onset of viral latency and a significant increase in plasma levels of viral RNA levels. The rapid expansion of HIV and the subsequent rise in viral RNA levels have substantial clinical implications, as they coincide with the irreversible destruction of helper T cell reservoirs and the establishment of viral latency, which can lead to further adverse consequences. The timeframe from the last known HIV exposure within the past 14 days to the initial clinic visit and the infection date was estimated to be 7 days prior to the appearance of skin lesions. The interval between the detection of p24 antigen and the presence of HIV antibodies is approximately 54 days. The duration of Fiebig II, a specific stage of early HIV infection, is slightly longer than previously reported in other studies (5,10). Patients with acute early infection pose a higher risk of transmission compared to those with established infection partly due to their high viral load. Early initiation of ART can potentially limit the size of the latent pool of HIV-infected CD4 T cells. It is recommended to choose an ART regimen with a high barrier resistance, such as an integrase inhibitor or protease inhibitor, for optimal suppression of HIV viral load. Compared to previous studies on the concurrent infection of MPXV and acute HIV infection (6–7), our patient diagnosed with mpox and acute HIV showed a relatively mild clinical course. After initiating ART for less than a month, the patient demonstrated improved HIV control and immune reconstitution. These findings highlight the importance of recognizing HIV infection among MSM presenting with MPXV infections, and the potential link between sexual practices and HIV acquisition.
-
Patients with suspected mpox should be tested for HIV, unless they have already been diagnosed, particularly if they have engaged in recent high-risk sexual behavior.
-
No conflicts of interest.
-
The team at the Clinical and Research Center for Infectious Diseases at Beijing Youan Hospital for their care during this outbreak period.
PUBLIC HEALTH RESPONSE
| Citation: |



 Download:
Download:




