-
In this report, the main platforms in Chinese Center for Diseases Control and Prevention for prion study and diagnosis were introduced. Some findings of cross-species transmission and some potential anti-prion candidates were also discussed. Several prion-infected rodent models based on wild-type and transgenic animals were developed. Two in vitro protein amplification methodologies, protein misfolding cyclic amplification (PMCA) and real-time quaking-induced conversion (RT-QuIC), were established and applied in many different prion studies, whilst RT-QuIC with cerebrospinal fluid and skin specimens was applied in the diagnosis of human prion diseases. PMCA can help prion strains overcome species barrier, efficiently propagating in vitro and inducing interspecies infection in vivo. Some natural components, such as resveratrol and 3,4-dihydroxybenzalacetone (DBL), showed anti-prion activities both in vitro and in vivo.
Establishment of many special platforms efficiently deepens the prion studies and provides new sensitive tools for the surveillance and diagnosis of human prion disease.
Prion infection shares some similarities with the infections of other microorganisms (e.g., viruses and bacteria), such as fulfilling Koch postulates, having strain features, showing species barriers, etc. On the other hand, prion infection has unique characteristics, such as not eliciting host specific humoral and cellular immunoresponses without pathogenic-associated nucleic acids, etc. (1).
Prion disease is also a zoonotic disease. Transmission across different species of animals may occur naturally and artificially. The most famous example is the outbreak of bovine spongiform encephalopathy (BSE) in cattle in the United Kingdom (UK) and other European countries in the 1980s’ caused by feed consisting of meat and bone meal (MBM) being contaminated with scrapie agents. Subsequently, a new type of human prion disease, variant Creutzfeldt-Jacob disease (vCJD), emerged in the UK and many other countries worldwide due to consuming BSE-contaminated beef (2).
Because of the uniqueness of prion biology and special physiopathology of prion disease, prion study and disease diagnosis usually need many special platforms and techniques that are different from classical microbes. Transgenic techniques supplied the basis for the development of prion theory and protein amplification techniques in vitro which accelerated the speed of prion research. In this report, the main platforms in Chinese Center for Diseases Control and Prevention for prion study and transmissibility of different prion strains in bioassays were summarized (Figure 1).
-
Small experimental rodents, e.g., mice and hamsters, are commonly used for some strains of prions. The first prion mouse model in China was imported and established by Prof. Tao Hung in the Institute of Virology, Chinese Academy of Preventive Medicine in the 1980s.
Afterwards, several scrapie infected rodent models have been established and the clinical, neuropathological, pathogenic, and infectious characteristics have been comprehensively analyzed, including a hamster infected with scrapie hamster-adapted strain 263K, a C57 mouse infected with scrapie mouse-adapted strain 139A, and a C57 mouse infected with scrapie mouse-adapted strain ME7. Despite different median infective dose and incubation times, the major neuropathological and pathogenic features of different inoculating ways were the same. Later, other scrapie mouse models were established, such as the C57 mouse infected with agent 22L, C57, Balb/c, and CD1 mouse infected with the lysate of prion infected SMB-S15 cells, and C57 mouse infected with PMCA product (3).
We have also imported or developed several transgenic (Tg) mouse strains, such as Tg mouse knockout prion protein gene (PRNP)
, Tg mouse with human PRNP, Tg mouse with hamster PRNP, etc. Recently, based on the Tg mouse of knockout PRNP, we established two strains of Tg mice expressing chimeric MiniSOG-MusPrP aiming to study the morphology of scrapie fibril agent (SFA) and a strain of Tg mouse expressing human PRNP with T188K mutant (4). The relevant studies are ongoing. -
PMCA is a technique by mixing scrapie-like prion protein (PrPSc) in the tissues of infected animals with cellular prion protein (PrPc) from normal brains under the condition of cyclic processes of alternative sonication and incubation in a special facility, a large amount of normal PrPc can be converted into PrPSc in two to four days. Two PMCA methods were developed in our laboratory, direct PMCA aiming to detect PrPSc in the tissue samples and serial PMCA aiming to continual passage of PrPSc in vitro. Compared with the routinely used Western blot, the detection sensitivity of PMCA for PrPSc in the brains of prion infected experimental rodents increased 102–103 folds (5).
PrPSc propagation was elicited in muscle and spleen tissues, which were approximately 10-2 lower than brains. Traces of PrPSc replication were also detected in the intestine and kidney tissues of infected hamsters, which were about 10-4 lower than brains.
Pyridine nucleotides, a group of coenzymes ubiquitous in biosynthesis and metabolism, are associated with the aggregation of recombinant prion protein (rPrP). By using PMCA, we confirmed that PrPSc from the scrapie infected rodent brains propagated much more efficiently in the presence of reduced pyridine nucleotides, nicotinamide adenine dinucleotide phosphate hydrogen (NADPH) and nicotinamide adenine dinucleotide hydrogen (NADH), but remained unchanged in the presence of oxidized form of pyridine nucleotides, nicotinamide adenine dinucleotide phosphate (NADP), nicotinamide adenine dinucleotide (NAD), and vitamin C. The enhancement of reduced pyridine nucleotides on PrPSc replication was also observed in the PMCA using recombinant hamster prion protein (PrP) as substrate. It supplies the molecular basis for the involvement of reduced pyridine nucleotides in prion replication (6).
-
Real-time quaking-induced conversion (RT-QuIC), has been developed and greatly improved the detection limit of PrPSc (7). Cerebral spinal fluid (CSF) RT-QuIC has been also set up in Chinese national surveillance (8-9). The 1st generation of RT-QuIC used the full-length recombinant hamster PrP (rHaPrP23-231) as the substrate and the 2nd generation used the truncated hamster PrP (rHaPrP90-231). Validation with clinical CSF samples of definite sporadic CJD (sCJD) patients with neuropathological diagnosis, the established RT-QuIC (the 2nd generation) showed good sensitivity (96.67%) and specificity (100%). Wide application of CSF RT-QuIC in CJD surveillance also revealed high positive rates in probable sCJD cases, making it acceptable in the national diagnostic criteria for sCJD (9). Two separate studies illustrated that the positive rates (30%–32%) of CSF RT-QuIC in genetic prion disease were generally lower than those of sCJD. Based on the data of five commonly identified genetic prion diseases in China, the positive rates from highest to lowest were P102L Gerstmann-Sträussler-Scheinker syndrome (GSS) (60%–61.5%), E200K genetic CJD (gCJD) (40%–44%), E196A gCJD (37.5%–40%), T188K gCJD (25.7%), and D178N fatal familial insomnia (FFI) (15.8%–16.2%) (10).
Recently, after collaborations with Prof. Zou from Case Western Reserve University, traces of prions in skin specimens of sCJD patients and scrapie infected rodents were verified to be sensitively detectable by RT-QuIC (11-12). Validation of skin RT-QuIC with dozens of skin specimens from sCJD cases and non-CJD patients showed 100% specificity and 95.5% sensitivity (13). Compared to brain biopsy and lumbar puncture, skin biopsy is much less invasive.
One of the neuropathological characteristics of FFI cases is the significantly lower amount of PrPSc in the brain tissues (14-15). Positive results of RT-QuIC were detected in the postmortem brain tissues of FFI cases at 10-5 dilution. The RT-QuIC reactivities (e.g., lag time and fluorescent peak) of FFI brains were significantly weaker than that of sCJD (16). High sensitivity and wide tissue adaption of RT-QuIC make it become a powerful diagnostic tool for prion disease and prion study.
-
Our proteomics assay of the CSF samples of sCJD revealed a significant increase of calmodulin (17). A later study confirmed that the expressions of calmodulin in the brains of scrapie infected rodents were also upregulated, highlighting the possibility of setting up a new tool for diagnosis of sCJD (18). Western blot positively identified CSF calmodulin in 70% (28/40) of probable sCJD and 73.3% (22/30) of pathologically definite sCJD, whilst in 22.5% (9/40) of non-CJD and 20% (6/30) of pathologically excluded non-CJD. Logistic regression established a significant correlation between the CSF calmodulin signal and total CSF tau level (19).
Six tau isoforms with different molecular weights have been found in the brains, differing by the number of exon-2 (29-aa) and/or exon-3 (29-aa) insertion in the N-terminus and exon-10 (31-aa) in the C-terminal half of the protein. We have separately prepared the specific polyclonal and monoclonal antibodies against exon-2, -3 and -10 of tau (20). A 65 kDa-large band was detected in the CSF samples of sCJD patients, in the reactions of the antibodies to exon-2 and exon-10, revealing good correlation between positive CSF 14-3-3 and typical abnormality in electroencephalogram (EEG). Majority of the increased tau in the CSF of sCJD cases was derived from the tau isoforms with exon-2 and exon-10 segments (21).
-
Species barriers of prion infection in experimental mice and hamsters show an interesting phenomenon. Intracerebral infections of mouse-adapted scrapie strains 139A and ME7 onto hamsters could cause typical prion disease after long incubation periods. Contrary to the short lag time of homologous infection of hamster adapted scrapie strain 263K (66–80 days), the hamsters with heterologous infection by strains of 139A and ME7 displayed typical diseases after 358–450 and 460–530 days, respectively. The molecular characteristics of the newly formed PrPSc in 139A and ME7 infected hamsters were obviously distinct from their original mouse ones, whilst greatly similar to that of hamster strain 263K. On the other hand, inoculation of the hamster-adapted scrapie strain to mice was unable to induce prion disease. Cross-species transmission of prions between mouse and hamster under the experimental condition was a one-way direction, transmissible from mouse to hamster but not transmissible from hamster to mouse (22).
PMCA verified that both mouse-adapted strain 139A and hamster-adapted strain 263K could use brain homogenates of opposite species to form PrPSc. These two newly formed interspecies PMCA prions could stably propagate in the subsequent serial PMCA passages; meanwhile, they lost their original molecular characteristics but possessed the new host features. Inoculations of new PMCA prions to the heterologous animals efficiently caused the typical prion diseases. Unlike the prolonged lag time of interspecies infection of mouse strain 139A on hamsters, the incubation time of the hamsters infected with 139A-induced interspecies PMCA prion was much shorter. This suggests that PMCA can help prion strains overcome species barrier, efficiently propagating in vitro and inducing interspecies infection in vivo (Figure 2) (23).
 Figure 2.
Figure 2.Schema of interspecies transmissions of mouse- or hamster-adapted scrapie prions onto opposite animals directly or via PMCA. Scrapie agents in the brain tissues of infected animals (brain-prion) or via PMCA amplification (PMCA-prion) were separately inoculated into respective animals intracerebrally.
Abbreviation: PMCA=protein misfolding cyclic amplification. -
Resveratrol (3,4',5-trihydroxy-trans-stilbene) is a natural polyphenolic phytoalexin from many fruits and vegetables, showing a number of health benefits, such as neuroprotection, cardioprotection, hepatoprotection, anti-inflammation, and cancer chemoprevention. Exposure of prion infected cell line SMB-S15 to resveratrol remarkably reduced and even removed cellular PrPSc in a dose-dependent manner. Prion replication in SMB-S15 cells treated with 5 and 10 μmol/L resveratrol was irreversible after the drug withdrawal. Inoculation of the lysates of resveratrol-treated SMB-S15 cells on mice completely lost the infectivity, proposing a valuable therapeutic potential (24).
Two other stilbene compounds, pterostilbene (Pte) and piceatannol (Pic), also revealed anti-prion activities in vitro study. In the level of cultured cells, obvious suppressions on PrPSc replication in SMB-S15 cells were observed, in which resveratrol (Res) was the most active one, followed by Pic and Pte. The inhibitive activities of those three stilbenes on the brain-derived prion from agent 263K-infected hamster were also identified in hamster PrP-based PMCA and RT-QuIC. Molecular binding of stilbene compounds with mouse PrP was proven by Biacore assays, highlighting an association between clearance of prions and molecular binding (25).
Anti-prion activity was also observed in other natural compounds, such as 3,4-dihydroxybenzalacetone (DBL), a small catechol-containing compound purified from the ethanol extract of Inonotus obliquus. After exposure to 10 μmol/L of DBL, the level of PrPSc in SMB-S15 cells was significantly decreased. The levels of reactive oxygen species and hydrogen peroxide were decreased, whereas the levels of some antioxidant factors, such as heme oxygenase 1, glutamate-cysteine ligase catalytic, and glutamate-cysteine ligase modifier, were significantly increased. The activities of total glutathione and superoxide dismutase, and the levels of unfolded protein response-related proteins were upregulated. Such anti-cytotoxicity phenomena were only detected in DBL-treated prion infected cells but not in their normal partner cells. Another compound, vanillic acid, also displayed similar anti-prion activity in vitro (Figure 3).
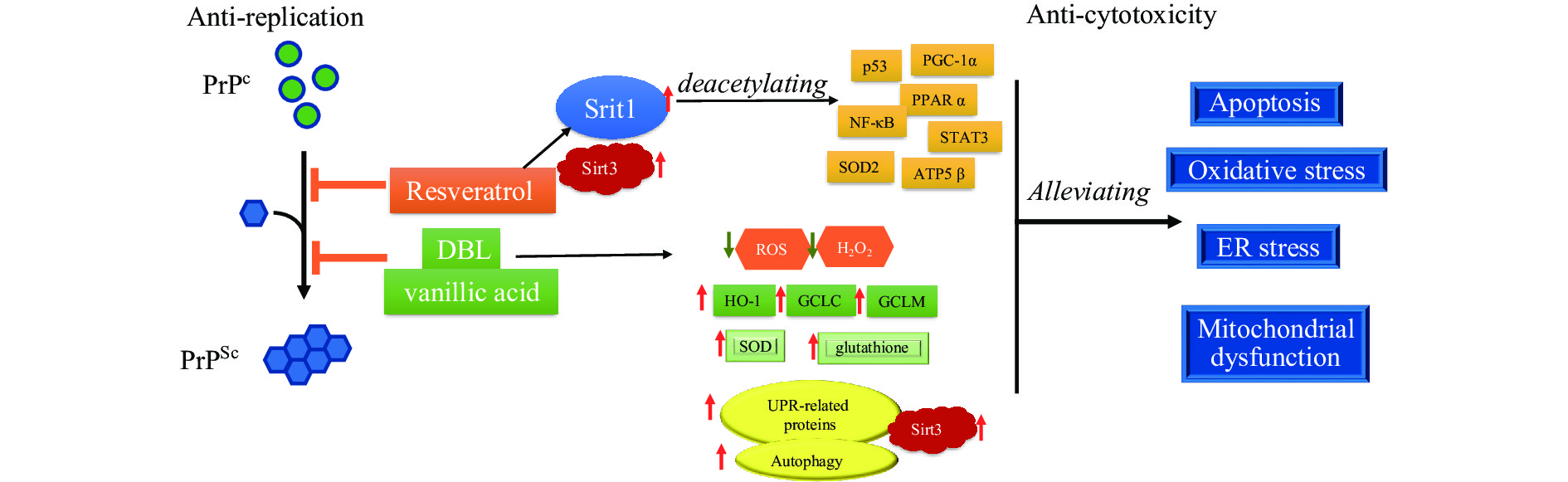 Figure 3.
Figure 3.Scheme of the activities of anti-prion replication and anti-prion cytotoxicity of resveratrol, 3,4-DBL, and vanillic acid. Resveratrol, DBL, and vanillic acid inhibit the replication and infectivity of prions via direct molecular interaction while alleviating the prion cytotoxicity by mediating various cellular agents and pathways.
Abbreviation: PrPSc=scrapie-like prion protein; PrPc=cellular prion protein; ROS=reactive oxygen species; SOD=superoxide dismutase; ER=endoplasmic reticulum;DBL=dihydroxybenzalacetone. -
Because of the unique biological features, study and diagnosis of prions and prion disease are completely different from other microorganisms and infectious diseases. Up to now, animal assays are still one of the most important platforms for prion study. Numerous types of transgenic mice including PRNP and other associated genes greatly help to understand the pathogenesis. PMCA techniques markedly increases the speed of prion replication, and PMCA-generated prions are usually infectious, showing a great advantage for evaluating prion infection. RT-QuIC usually reflects the fiberizing ability of prion, and its product usually does not have infectivity. Thus, animal bioassays are still an indispensable tool for prion research.
Neuropathological and PrPSc tests based on postmortem brains are the pathway for definite diagnosis of human prion diseases. However, brain tissues obtained from biopsy or autopsy have been limited since the establishment of the surveillance network. In addition to the traditional culture of the Chinese people, fear of prion infectivity is another reason for refusing to perform biopsies or autopsies in hospitals. In the future, publishing guidelines for biopsies or autopsies of sCJD patients will be helpful to obtain brain tissues. In the meantime, verifying the accuracy and reliability of skin RT-QuIC assay and applying skin RT-QuIC assay to partially replace brain tissue pathology is also an ideal option in China.
For decades, screening of specific biomarkers in CSF and other peripheral specimens has been continually conducted. Only two CSF proteins, 14-3-3 and tau, are included in the diagnostic criteria. However, the diagnostic sensitivity and specificity of those two proteins in CSF vary largely among the different laboratories. Further searching of the diagnostic biomarkers in other easily obtained samples. i.e., blood and urine, with novel techniques is valuable. The diagnostic significance of CSF RT-QuIC for sCJD has been documented. More recently developed skin RT-QuIC reveals even better diagnostic sensitivity and specificity for sCJD. Further standardization and industrialization of RT-QuIC will improve its usage clinically.
The outbreak of BSE and emergence of vCJD alarm the possibility of the transmission of prion strains from other species of animals to humans. Chronic wasting disease is considered as also having potential. The mechanism of cross-species transmission of prions is one of the hot topics in the field of prion study. Studies based on PMCA have verified that PMCA can help prion strains to transmit interspecies and even generate new prion strains de novo, highlighting that prions affecting new susceptible species may be generated by artificial extreme environments, such as the production process of meat and bone meal. PMCA, RT-QuIC, and other new methodologies supply useful tools for fast screening of potential materials for research and development of therapeutic and prophylactic drugs. However, to date, no specific, effective prophylactic regimens or therapeutic tools have been established for human or animal prion diseases, which still needs more effort.
-
All staffs, doctoral and master students who have worked and studied in the Department of Prion Disease of National Institute for Viral Disease Control and Prevention, Chinese Center for Diseases Control and Prevention since 1998, and the colleagues from provincial CDCs and sentinel hospitals who have participated in the works in National Surveillance for prion diseases since 2006.
HTML
| Citation: |



 Download:
Download:




