-
The coronavirus disease 2019 (COVID-19) pandemic remains a global public health crisis, with nearly half a billion cases and over six million deaths reported to the World Health Organization (WHO). Vaccines are one of the most effective tools against COVID-19. More than 11 billion doses have been administered globally, and 64% of the world’s population has received at least 1 dose of a COVID-19 vaccine (1). On December 15, 2020, China initiated a COVID-19 vaccination campaign. More than 3.2 billion doses have been administered, the vast majority of which have been inactivated vaccines.
Studies have demonstrated good effectiveness of COVID-19 vaccines made using different technical platforms on prevention of severe illness and death, effectiveness at preventing infection is much less. Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) has been evolving, and the variants seen in China have paralleled strains seen globally. Omicron has now replaced Delta to become the predominant strain in China.
Protective effectiveness of vaccines produced and used in China against different variants must be monitored carefully. Using individual-level matched data from two databases — the National Notifiable Disease Reporting System (NNDRS) that records clinical information on COVID-19 patients and the National Immunization Program Information System (NIPIS) that record vaccination histories — we analyzed clinical outcomes of Delta and Omicron infections by vaccination status to estimate protective effectiveness of COVID-19 vaccines used in China.
This was a retrospective, descriptive analysis based on all cases of COVID-19 diagnosed nationwide in China between May 21, 2021 and February 28, 2022. We estimated the effectiveness of vaccination to prevent progression of illness by comparing the odds of vaccination in different outcomes using an age-stratified analysis.
This study included all individuals diagnosed with COVID-19 in the mainland of China reported to NNDRS between May 21, 2021 and February 28, 2022. Cases in NNDRS were matched by national identification number to vaccination histories in NIPIS.
Vaccination status was categorized into four groups: unvaccinated, partially vaccinated, full vaccination, and booster vaccination. The unvaccinated group consisted of individuals who did not receive any COVID-19 vaccines before becoming infected or received COVID-19 vaccines on the day of infection. The partially vaccinated group consisted of individuals who had not completed full primary vaccination or who completed primary vaccination less than 14 days before becoming infected. The full vaccination group consisted of individuals who completed 2 doses of inactivated vaccine, 1 dose of adenovirus-vectored vaccine, or 3 doses of recombinant-subunit vaccine 14 or more days before becoming infected. The booster dose group consisted of individuals who received a booster dose 7 or more days before becoming infected. Breakthrough cases were individuals who completed full vaccination or booster vaccination before becoming infected.
According to the Protocol for Prevention and Control of COVID-19 (Eighth Version) (2), cases are classified by clinical symptoms as asymptomatic, mild, moderate, serious, or severe. We categorized cases into one of two outcomes: pneumonia, which includes moderate, serious, and severe cases; and serious COVID-19, which included only serious and severe cases.
The study used SPSS software (version 25.0, IBM Crop., Armonk, NY, US) to perform the following tasks: (a) descriptive analysis of the distributions of clinical outcomes by age group, vaccination status, and variant, (b) variant-specific, age-stratified, univariate logistic regression analyses of COVID-19 pneumonia cases and exact logistic regression of serious COVID-19 cases by vaccination category among cases ≥3 years old to determine odds ratios (OR) and their 95% confidence intervals (CI). We calculated effectiveness of vaccination to prevent COVID-19 pneumonia or serious COVID-19 as one minus the ORs times 100%. Statistical significance was considered as P<0.05.
A total of 10,829 domestic COVID-19 cases were reported between May 21, 2021 and February 28, 2022 in the mainland of China. Among those, 8,675 were caused by the Delta variant and 2,154 were caused by Omicron. Vaccination status by age group and variant is shown in Figure 1. Among cases ≥3 years, 15.1% were unvaccinated, 7.3% were partially vaccinated, 63.6% were fully vaccinated, and 14.0% received a booster dose.
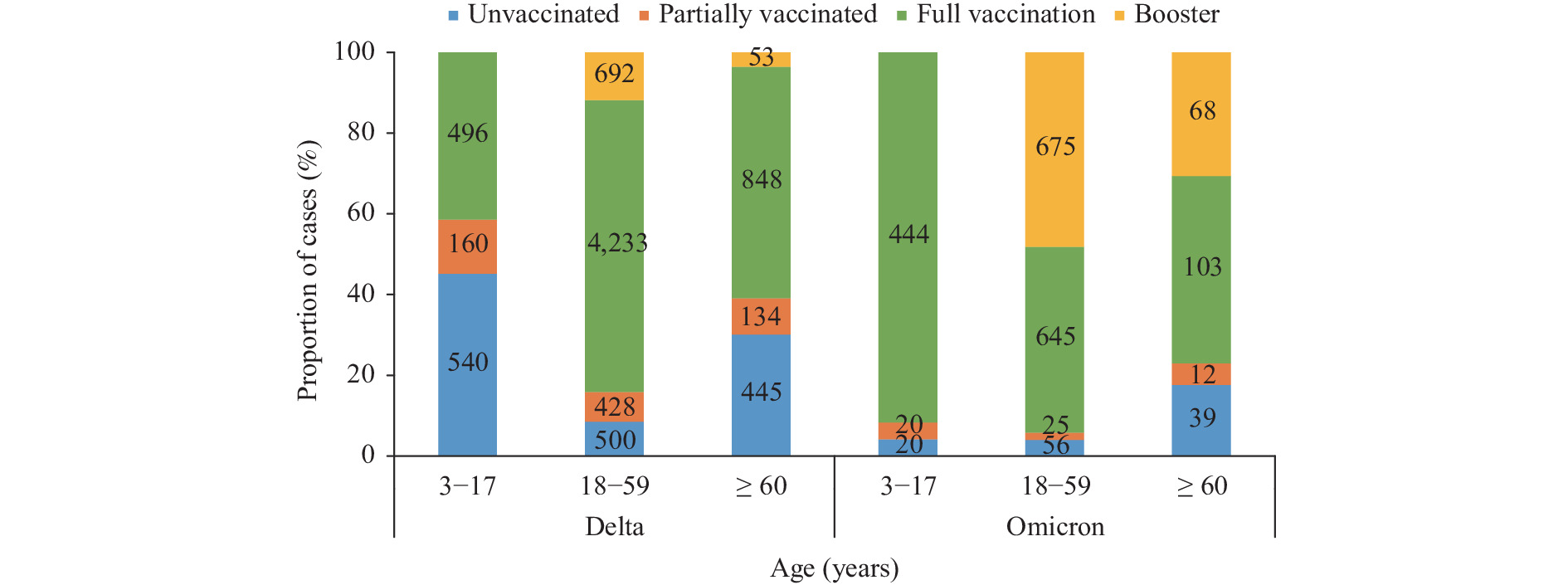 Figure 1.
Figure 1.Age-specific COVID-19 vaccination status among Delta- and Omicron-infected cases, China, May 21, 2021–February 28, 2022.
Among cases with full vaccination, 95.1% (7,849) received inactivated vaccine, 2.2% (183) received adenovirus-vectored vaccine and 2.7% (223) received recombinant-subunit vaccine. Among Delta cases, the proportions of fully vaccinated cases with COVID-19 pneumonia in inactivated vaccine, adenovirus-vectored vaccine, and recombinant-subunit vaccine groups were 95.9% (3,197), 1.5% (50), and 2.6% (85), while the proportions of serious cases were 97.5% (157), 0 (0), and 2.5% (4), respectively. Among Omicron cases, the proportions of fully vaccinated cases with COVID-19 pneumonia in inactivated vaccine, adenovirus-vectored vaccine, and recombinant-subunit vaccine groups were 90.9% (497), 6.2% (34), and 2.9% (16) respectively, and only the inactivated vaccine group had 9 serious cases.
Table 1 shows outcomes by variant and age group among the 1,793 unvaccinated cases (1,631 Delta and 162 Omicron); 59.0% of unvaccinated Delta cases had pneumonia and 37.7% of unvaccinated Omicron cases had pneumonia; 10.6% of unvaccinated Delta cases were severe, and 2.5% of unvaccinated Omicron cases were severe (P<0.01). Overall, Omicron cases were 68% (95% CI: 52%–79%) less likely to develop pneumonia and 80% (95% CI: 43%–94%) less likely to become severe, a finding that held for both adult groups.
Age
(years)Variants Number of
cases (N)COVID-19 pneumonia cases Serious COVID-19 cases n (%) P value OR (95% CI) n (%) P value OR (95% CI) <3 Delta 146 28(19.2) Ref Ref 0(0) − − Omicron 47 10(21.3) 0.75 1.14(0.51–2.56) 0(0) − − Subtotal 193 38(19.7) 0(0) 3–17 Delta 540 169(31.3) Ref Ref 1(0.2) Ref Ref Omicron 20 2(10.0) 0.06 0.24(0.06-1.06) 0(0) 0.96 − Subtotal 560 171(30.5) 1(0.2) 18–59 Delta 500 363(72.6) Ref Ref 39(7.8) Ref Ref Omicron 56 18(32.1) <0.01 0.18(0.10–0.32) 0(0) 0.03 0.15(0–0.68) Subtotal 556 381(68.5) 39(7.0) ≥60 Delta 445 402(90.3) Ref Ref 133(29.9) Ref Ref Omicron 39 31(79.5) 0.04 0.41(0.18–0.96) 4(10.3) <0.01 0.27(0.07–0.77) Subtotal 484 433(89.5) 137(28.3) Total Delta 1,631 962(59.0) Ref Ref 173(10.6) Ref Ref Omicron 162 61(37.7) <0.01 0.32(0.21–0.48) 4(2.5) <0.01 0.20(0.06–0.57) Subtotal 1,793 1,023(57.1) 177(9.9) Note: –: data not applicable.
Abbreviations: Ref=reference; OR=odds ratio; CI=confidence interval.Table 1. Age- and variant-specific clinical outcomes among unvaccinated cases, China, May 21, 2021–February 28, 2022.
Table 2 shows age-stratified logistic regression analyses of the outcomes by vaccination status separately for Delta cases and Omicron cases. Among Delta cases, the proportions of fully vaccinated cases with COVID-19 pneumonia in the 3–17 year, 18–59 year, and ≥60 year groups were significantly lower than respective proportions in the unvaccinated groups. By age group, the risk of developing pneumonia was 58%, 51%, and 66% lower in the full vaccination age groups compared with no vaccination. The proportions of fully vaccinated serious cases in the 18–59 year and ≥60-year age groups were lower than age-group-respective unvaccinated cases; full vaccination was associated with 83% and 69% lower risk of becoming severe.
Variants Age
(years)Vaccination Number of cases COVID-19 pneumonia cases Serious COVID-19 cases N n (%) P value OR (95% CI) n (%) P value OR (95% CI) Delta 3–17 Unvaccinated 540 169(31.3) Ref Ref 1(0.2) Ref Ref Partially vaccinated 160 31(19.4) <0.01 0.53(0.34–0.81) 0(0) − − Full vaccination 496 80(16.1) <0.01 0.42(0.31–0.57) 0(0) − − Subtotal 1,196 280(23.4) 1(0.1) 18–59 Unvaccinated 500 363(72.6) Ref Ref 39(7.8) Ref Ref Partially vaccinated 428 286(66.8) 0.06 0.76(0.57–1.01) 12(2.8) <0.01 0.34(0.16–0.68) Full vaccination 4,233 2,392(56.5) <0.01 0.49(0.40–0.60) 59(1.4) <0.01 0.17(0.11–0.26) Booster 692 185(26.7) <0.01 0.14(0.11–0.18) 1(0.1) <0.01 0.02(0.01–0.10) Subtotal 5,853 3226(55.1) 111(1.9) ≥60 Unvaccinated 445 402(90.3) Ref Ref 133(29.9) Ref Ref Partially vaccinated 134 126(94.0) 0.19 1.69(0.77–3.68) 22(16.4) <0.01 0.46(0.27–0.77) Full vaccination 848 645(76.1) <0.01 0.34(0.24–0.48) 99(11.7) <0.01 0.31(0.23–0.42) Booster 53 30(56.6) <0.01 0.14(0.07–0.26) 2(3.8) <0.01 0.09(0.01–0.36) Subtotal 1,480 1,203(81.3) 256(17.3) Omicron 3–17 Unvaccinated 20 2(10.0) Ref Ref 0(0) Ref Ref Partially vaccinated 20 4(20.0) 0.36 2.34(0.38–14.57) 0(0) − − Full vaccination 444 59(13.3) 0.63 1.44(0.32–6.37) 0(0) − − Subtotal 484 65(13.4) 0(0) 18–59 Unvaccinated 56 18(32.1) Ref Ref 0(0) Ref Ref Partially vaccinated 25 3(12.0) 0.07 0.29(0.08–1.10) 0(0) − − Full vaccination 645 203(31.5) 0.91 0.97(0.54–1.74) 3(0.5) 0.78 − Booster 675 192(28.4) 0.57 0.84(0.47–1.52) 2(0.3) 0.83 − Subtotal 1,401 416(29.7) 5(0.4) ≥60 Unvaccinated 39 31(79.5) Ref Ref 4(10.3) Ref Ref Partially vaccinated 12 7(58.3) 0.15 0.36(0.09–1.46) 0(0) 0.66 0.58(0–3.66) Full vaccination 103 54(52.4) 0.01 0.28(0.12–0.68) 4(3.9) 0.29 0.36(0.06–2.02) Booster 68 39(57.4) 0.02 0.35(0.14–0.87) 0(0) 0.03 0.10(0–0.61) Subtotal 222 131(59.0) 8(3.6) Note: –: data not applicable.
Abbreviations: Ref=reference; OR=odds ratio; CI=confidence interval.Table 2. Logistic regression analysis on risk of developing into COVID-19 pneumonia and serious COVID-19 by vaccination status, China, May 21, 2021–February 28, 2022.
Among Omicron cases ≥60 years old, the risk of developing into pneumonia was 72% lower than among unvaccinated cases, and there were no statistically significant differences in serious infections by full vaccination status.
At the time of the study, booster doses were recommended only for adults. Among 18–59 year and ≥60-year Delta cases, the booster dose had 86% and 86% lower risk of developing pneumonia. Compared with no vaccination age groups, risk of serious COVID-19 in the 2 adult booster age groups was 98% and 91% lower, respectively.
Among Omicron cases ≥60 years old, the booster dose was associated with a 65% decrease in pneumonia. There were no severe Omicron cases in the booster group ≥60 years old, while 4 (10.3%) of the unvaccinated cases in this age group were severe, the risk of developing into serious COVID-19 was 90% lower than among unvaccinated cases.
-
This study used national COVID-19 surveillance and vaccination data from May 21, 2021 through February 28, 2022 to estimate effectiveness of the China-produced vaccines that are building population immunity against SARS-CoV-2 and its variants. Based on individual-level analysis of all Delta and Omicron infections in the mainland of China, we found that full primary vaccination was associated with a 50%−70% lower risk of Delta-variant COVID-19 pneumonia in the vaccine-eligible age range (≥3 years), a 70%−80% lower risk of developing into serious Delta-variant COVID-19 among adults ≥18 years, and a 70%−80% lower risk of developing Omicron-variant pneumonia among adults ≥60 years. Booster doses have been recommended since October 2021 for adults ≥18 years. Among Delta infections, receipt of a booster dose was associated with an 86% lower risk of pneumonia and a 91%–98% lower risk developing serious COVID-19. Among Omicron infections in people ≥60 years, receipt of a booster dose was associated with a 65% lower risk of pneumonia. There were no serious Omicron cases among ≥60-year-olds who received a booster dose. Delta infections were more severe on average than Omicron infections in an unvaccinated population.
Because of China’s policy of “zero tolerance for local transmission”, there have been few domestic cases of COVID-19 and virtually all of China’s population immunity comes solely from vaccine, rather than hybrid immunity from infection and vaccine. The combination of increasing vaccination coverage and increased transmissibility of Delta and Omicron variants is causing a rapid increase of breakthrough cases of COVID-19. The degree to which vaccines maintain protection is critically important in the assessment of the strength of population immunity and is of great significance for adjustment of immunization strategies.
Compared with the international mRNA and adenovirus-vectored vaccines, there were fewer real-world data about breakthrough cases from China-produced COVID-19 vaccines. A case-control study in the US showed that full mRNA vaccination significantly reduced risk of hospitalization (OR=0.15) and death or invasive mechanical ventilation in hospitalized cases (OR=0.33) (3), findings consistent with ours. Our finding that China-made COVID-19 vaccines reduced risk of pneumonia by 72% among ≥ 60-year-olds infected with Omicron, with no serious boosted cases, was consistent with the results reported by the University of Hong Kong (4). Their study, which was also in an infection-naive population, showed that protective effectiveness of 2 doses of inactivated vaccine among people ≥ 60 years was 72% and protective effectiveness of a homologous booster dose was 98%. Although with a smaller number of serious cases, our study found a similar booster dose effect among ≥60-year-olds, with a 90% reduction of serious COVID-19 caused by the Omicron variant. We need to sustain monitoring and analysis of breakthrough cases for more refined assessments.
Existing evidence from neutralizing antibody testing shows that the Omicron variant has partial immune escape (5). A real-word study in South Africa found that the protective effect of BNT162b2 vaccine against Delta was 93% after full vaccination, but decreased to 70% against Omicron. Although effectiveness of COVID-19 vaccines against infection with Omicron is lower, protection against hospitalization and death remains high (6). Data from U.S. CDC compared unvaccinated, full primary vaccinated, and boosted individuals, and found significantly reduced morbidity (725.6/100,000, 254.8/100,000 and 148.6/100,000) and mortality (7.8/100,000, 0.6/100,000 and 0.1/100,000) by vaccination and boosting (7).
This study was subject to several limitations. First, the clinical outcomes (severity) of cases were based on the most severe clinical classification reported, which may cause some misclassification bias due to regional variation in classification. Second, the study could only analyze associations between vaccination and clinical outcomes, and we did not have data on underlying medical conditions and did not control for time interval after vaccination and other factors associated with protective effectiveness. Third, our study was a case-case study, a special kind of case-control study, so the results only reflected the association between vaccination and risk of disease development and were not direct measures of vaccine effectiveness. Moreover, in view of the vast majority of cases administered inactivated vaccines and the case sizes of the other two vaccines were not big enough to do statistical analysis, our study did not include the analysis of different technical platforms vaccines.
In conclusion, our study of Delta infections showed that China-made COVID-19 vaccines were associated with a 50% to 70% lower risk of developing into pneumonia among all ages and a 70% to 80% lower risk of severe COVID-19 among adults. Booster doses were associated with an 86% risk reduction for pneumonia and a 91% to 98% risk reduction of developing severe COVID-19. Our study of Omicron infections found that full vaccination and booster doses were associated with reduced risk of pneumonia among adults over 60 years, with the booster dose associated with reduced risk of developing serious COVID-19. In an unvaccinated population, Delta infections were more severe on average than Omicron infections.
HTML
| Citation: |



 Download:
Download:




