-
Surveillance in Jinzhai County, Lu’an City, Anhui Province, China revealed that between May and June 2021, 2 patients developed fever and thrombocytopenia after being bitten by a tick. The local hospitals and CDC could not determine the pathogen, so blood specimens were sent to the National Institute for Communicable Disease Control and Prevention to investigate this event. Nested polymerase chain reactions (PCR) and indirect immunofluorescence assays (IFAs) identified Anaplasma bovis as the pathogen of both cases. Gene sequencing suggested that a tick, Haemaphysalis longicornis, was the source of infection. This is the second report of A. bovis infections in humans worldwide. Clinicians and public health physicians should be more attentive to these diseases and learn how to diagnose them as early as possible.
-
In 2021, 2 patients (Patient A and Patient B) developed intermittent fever as an initial clinical symptom after a tick bite. Both of them were characterized by fever, thrombocytopenia, rash, asthenia, anorexia, myalgia, chill, diarrhea, and headache, which were not resolved by common antibiotics. In a local hospital, these patients were clinically diagnosed with endemic typhus.
On May 10, 2021, a 67-year-old man (Patient A) developed a fever with chills, asthenia, anorexia, and myalgia after being bitten by a tick in a mountainous area. Even with routine anti-infection treatments, his body was covered with a rash 3 days later. He was brought to the Department of Infectious Disease of Jinzhai County People’s Hospital (JCPH; Lu’an City, Anhui Province, China) on May 17, 2021, with persistent intermittent fever and rashes. Based on his medical history and epidemiological and clinical manifestations, he was admitted to JCPH with clinically diagnosed typhus. Blood tests revealed eosinophilic granulocytes of 0.00 × 109/L, thrombocytes of 87 × 109/L, alanine aminotransferase of 117.07 U/L, and aspartate transaminase of 104.13 U/L. After treatment with azithromycin for 7 days, his clinical symptoms disappeared.
On June 5, 2021, a 57-year-old man (Patient B) developed a fever reaching 39 °C accompanied by headache, asthenia, anorexia, myalgia, and occasional diarrhea after working in a field. He was brought to the Department of Infectious Disease of JCPH on June 8, 2021, with progressive exacerbation of his symptoms. He was admitted to JCPH with “fever of undetermined origin,” and his general status upon hospital admission was poor. Blood tests revealed leukocytes of 3.94 × 109/L and thrombocytes of 95 × 109/L. After treatment with azithromycin for 7 days, his clinical symptoms disappeared, and his general status improved greatly.
The blood specimens collected from these two patients were sent to determine the pathogen through nested PCR and IFA. DNA was extracted with the QIAamp DNeasy Blood & Tissue Kit (Qiagen, Hilden, Germany), and several human pathogens were tested using nested PCR targeting the 16S ribosomal RNA (rrs) gene. Specific immunoglobulin IgG and IgM antibodies against spotted fever group and related rickettsia (Rickettsia typhi, Orientia tsutsugamushi, A. phagocytophilum, Ehrlichia chaffeensis, and A. bovis in sera) were detected by IFA (Fuller Laboratories, Fullerton, CA, USA). The A. bovis substrate slides were developed by our department. According to the manufacturer’s instructions, an IgG titer of ≥1∶64 and IgM titer of ≥1∶20 denoted a positive result.
To investigate the presence of infections in relevant ticks, we collected parasitic and free-living ticks from the regions where the two patients lived and worked. All ticks were identified morphologically by an entomologist based on differences in their bodies and basis capituli. All tick DNA was extracted individually. We tested for the presence of Rickettsiales bacteria using a nested or semi-nested PCR targeting the rrs, citrate synthase (gltA), and 60-kDa heat shock protein (groEL) genes as described previously (Supplementary Table S1)(1). Phylogenetic trees of the data were created using the Maximum Likelihood (ML) method by employing the GTR+Γ+I model of substitution, as implemented in PhyML (version 3.0) http://www.atgc-montpellier.fr/phyml/ (2).
The rrs gene was amplified from Patient A using a PCR assay, while it was not detected from Patient B. Using BLASTN with a nucleotide collection, genetic analysis of the recovered rrs sequence revealed that it was most closely related to that of the A. bovis isolate Zhouzhi-cattle-10 (GenBank: MH255937.1, 99.75%), and we named it “A. bovis strain JZPA.” The IgM titer against A. bovis of these 2 patients was 1∶80, whereas the IgG titer of Patient A was 1∶256, and that of Patient B was 1∶1,024 (Figure 1). Therefore, PCR and IFA indicated that both patients had been infected with A. bovis.
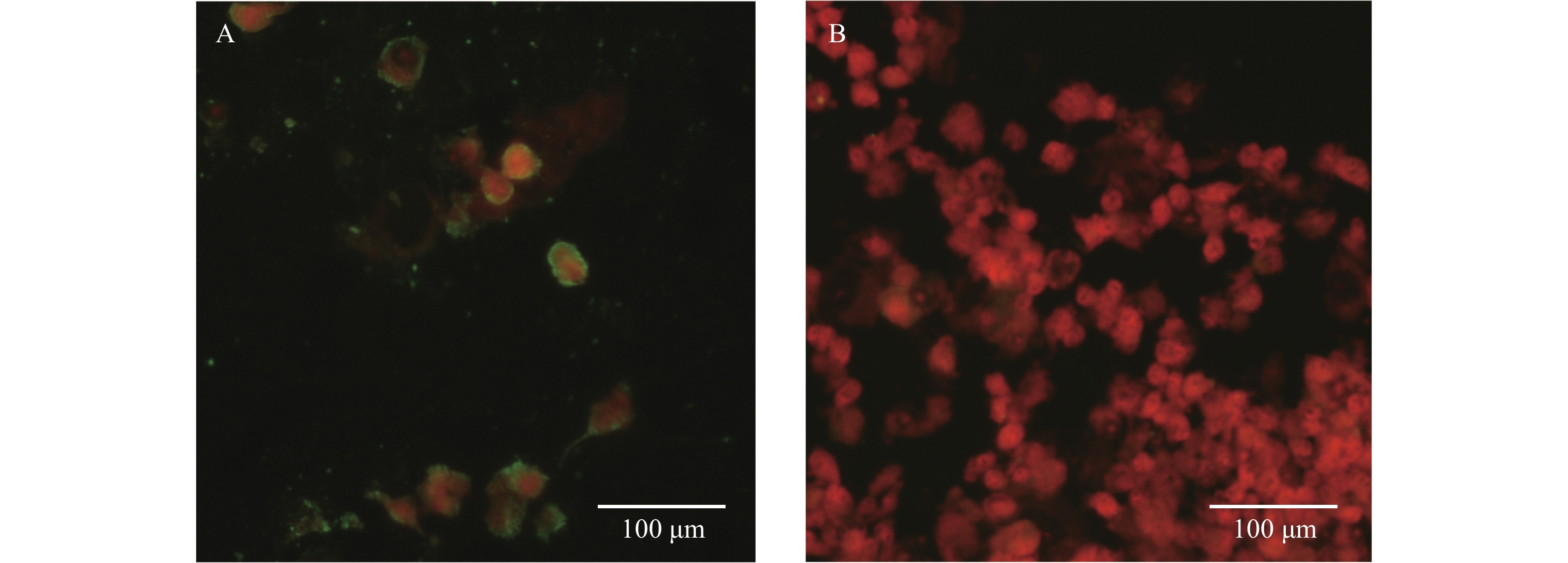 Figure 1.
Figure 1.Photomicrographs of an IFA test with Anaplasma bovis infected patients’ sera. (A) IFA of patient sera; (B) The IFA assays tested with negative control.
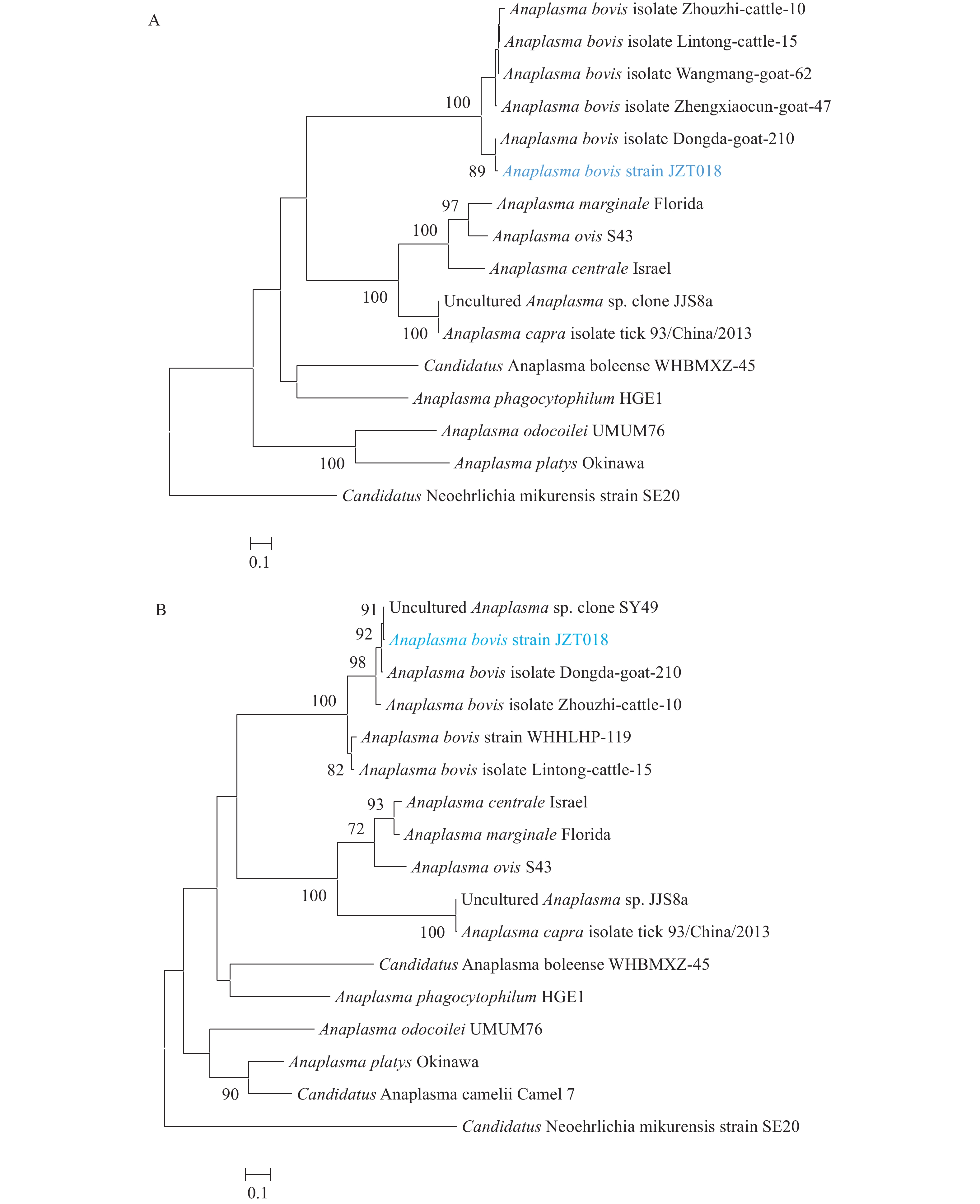
Note: Green fluorescence shows the positive cells for A. bovis bacteria, and red fluorescence shows the host cells. Abbreviation: IFA=immunofluorescence assay.To investigate the source of this pathogen, we carried out an investigation into the vectors present in the areas where the two patients lived and worked. We collected 270 tick samples, which were tested for the A. bovis strain JZPA. The rrs, gltA, and groEL genes were detected in 63 samples, and the positive rate in ticks was 23.3%. Each of the 63 samples contained all the three genes. In the rrs tree (Figure 2), sequences from Patient A’s sample (A. bovis strain JZPA) and sequences of the tick (A. bovis strain JZT018) clustered together and formed a distinct lineage. There was 100% similarity of the rrs gene sequences for the patient and the tick (H. longicornis). In the gltA and groEL trees (Supplementary Figure S1), the amplified tick sequences also clustered with A. bovis, and they were closely related to sequences from other A. bovis strains. These results suggested that A. bovis was prevalent in ticks within certain areas of Lu’an City and that these pathogens may infect humans to cause fevers and other symptoms (Table 1).
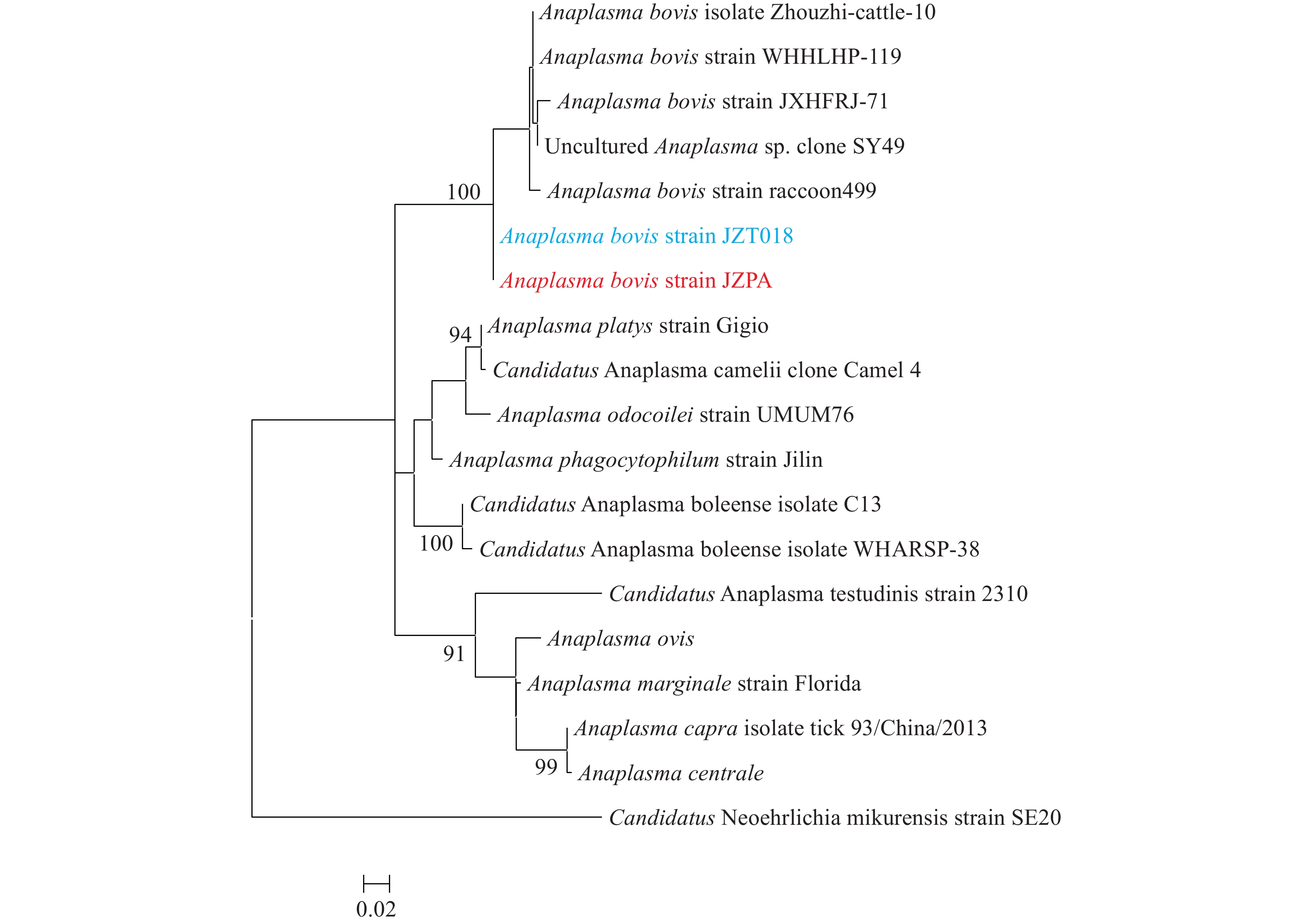 Figure 2.
Figure 2.Phylogenetic relationship of Anaplasma species based on rrs sequences.
Note: All trees were mid-point rooted for clarity only. Bootstrap values (>70%) were shown for appropriate nodes. Scale bar represents number of nucleotide substitutions per site. The sequence obtained from Patient A is marked in red, and the sequence obtained from ticks is marked in blue.Item Patient A Patient B Clinical characterization Fever + + Rash + − Asthenia + + Anorexia + + Myalgia + + Chill + − Headache − + Dizziness − − Nausea − − Lymphadenopathy − − Vomiting − − Diarrhea − + Eschar − − Cough − − Arthralgia − − Laboratory findings* White blood cell count 6.41×109/L 3.94×109/L Platelet count 87×109/L 95×109/L CRP 62.75 mg/L 4.93 mg/L ALT 117.07 U/L 14 U/L AST 104.13 U/L 24 U/L *Normal ranges: white–cell count: 4.0–10.0 × 109 /L, platelet count: 100–300 × 109 /L, CRP 0–8 mg/L, ALT: 0–40 U/L, AST: 5–40 U/L.
Abbreviations: CRP=C-reaction protein; ALT=alanine aminotrans
ferase; AST=glutamic oxaloacetic acid transferase.Table 1. Clinical manifestations and laboratory findings of two patients infected with Anaplasma bovis in Jinzhai County, Anhui Province, China, 2021.
-
Rickettsiales are obligate intracellular microbes responsible for a wide range of important human diseases, including anaplasmosis, ehrlichiosis, rickettsioses, and scrub typhus. Rickettsial diseases are not being effectively controlled worldwide (3). Several Anaplasma species, such as A. phagocytophilum, A. capra, and A. bovis, are considered human pathogens (1,4-5). Incidences of human granulocytic anaplasmosis have increased steadily since its discovery in the 1990s (4). A second Anaplasma spp., A. capra, was determined to be a human pathogen in 2015 (5). In Jiangxi Province in 2017, a patient reportedly became infected with A. bovis, which was previously considered to be a bovine-specific pathogen (1). In recent decades, novel pathogens have been increasingly identified due to the application of molecular diagnostic methods (6). Furthermore, numerous bacteria previously considered non-pathogenic are now commonly associated with human diseases (7). Because commonly administered antibiotics are ineffective for anaplasmosis, early diagnosis is important. If the identity of the pathogens is not promptly and accurately determined, these diseases may become severe and lead to death.
Both patients lived in the same township close to Jinzhai County, and neither had a history of traveling. We defined A. bovis as the cause of the illness based on PCR, IFA, and tick investigations. Unfortunately, the pathogens of A. bovis were isolated from ticks, but not from patients. This is the second report of A. bovis-infected humans worldwide, with the first being reported by Lu et al. in Jiangxi Province, China (1). Although the clinical symptoms were atypical, clinicians should pay attention to patients experiencing intermittent fever as an initial clinical symptom and thrombocytopenia after a tick bite or exposure to wildlife. Patients exhibiting these features should be considered to potentially have A. bovis infection.
Currently, the genus Anaplasma contains seven recognized bacterial species (6), most of which are known to be animal-specific pathogens; for example, A. marginale, A. centrale, and A. ovis are specific to ruminants; A. platys is a causative agent of infectious cyclic thrombocytopenia in dogs and cats (6); and A. bovis was thought to only infect bovine to cause bovine ehrlichiosis, which frequently occurs in Africa and Asia, until the first case was reported in humans. Animals infected with A. bovis are characterized by fluctuating fever, lymphadenopathy, depression, and occasionally death. When humans are infected with these pathogens, they display fever, rash, asthenia, anorexia, rigor, headache, myalgia, eschar, and lymphadenopathy.
A novel tick-borne bunyavirus was first discovered in Hubei and Henan provinces in 2009 (8), and human granulocytic anaplasmosis was reported in these regions (9). Bunyavirus and human granulocytic anaplasmosis infections can elicit similar symptoms, so distinguishing between them by using clinical manifestations can be difficult. For these patients, we used PCR and IFA to exclude the diagnoses of severe fever with thrombocytopenia syndrome and infection with other types of bacteria of the order Rickettsiales, and the results showed that both patients were infected with A. bovis.
We screened 13 patients with Rickettsiae and Anaplasma infection symptoms, and only 2 patients were determined with A. bovis infection. Although surveillance found only two patients in this study, these patients became sick within one month of each other and lived in the same township. The close timing of their illness, their adjacent place of residence, and the high prevalence rate of A. bovis in ticks near their work and living places suggest that more A. bovis-infected patients may exist in this area.
Human infections with several types of tick-borne pathogens in mainland China have been documented in recent years, and they are an increasing threat to public health (10). Missed diagnoses and misdiagnoses can lead to poor outcomes or even death, so an early and specific diagnosis is important. The recommendations from this case study are the following: 1) a rapid detection method for diagnosis (e.g., nested PCR or real-time PCR) should be established; 2) the high prevalence rate of A. bovis in ticks suggests that potential outbreaks may occur in this region, and more investigations should be conducted in arthropods and wild animals in these areas; and 3) people should be educated on how to protect themselves against tick bites, especially those in susceptible populations (e.g., farm workers).
-
No conflicts of interest declared.
Gene Name Primers (5'–3') Fragment size rrs fd1 AGAGTTTGATCCTGGCTCAG 1,500 bp rp2 ACGGCTACCTTGTRACGACTT groEL bovis-groF1 GTATGCARTTTGATCGYGGAT 1,330 bp bovis-groF2 GAAGTTGGAAGRGAYGGDGT bovis-groR GCCTTWACAGCDGCAACTTG 1,300 bp gltA bovis-gltA-F1 TACATCWACWGTAAGAATGG 1,100 bp bovis-gltA-F2 ACWGTAAGAATGGTKGGCTC bovis-gltA-R CCRGCAGTDCGTCCCAGTGC 1,057 bp COI Ron GGAGCYCCWGATATAGCTTTCCC 488 bp Nancy CCTGGTAAAATTAAAATATAAACTTC Abbreviations: PCR=polymerase chain reaction; rrs=16S ribosomal RNA; groEL=60-kDa heat shock protein; gltA=citrate synthase; COI=cytochrome-cytochrome oxidase. Table S1. The PCR primers used in this study.
HTML
| Citation: |



 Download:
Download: