-
Hepatitis A is an infectious liver disease caused by hepatitis A virus (HAV) that results in mild to severe illness. HAV is spread to immunologically naïve persons who ingest food or water contaminated with feces from HAV-infected individuals (1). Hepatitis A is often seen in sporadic cases, but is also seen in large outbreaks, such as the 1988 outbreak in Shanghai that caused over 300,000 cases and huge economic losses (2). In the pre-vaccine 1970s, China was highly endemic for HAV with a seroprevalence due to infection of 85%–95% in children aged 10–15 years. With China’s socioeconomic development and improvements in standard of living through the 1990s, HAV exposure and hepatitis A incidence shifted from a younger population to an older population (3). Following hepatitis A (HepA) vaccine development, licensure, and widespread introduction, China made great progress in hepatitis A control and prevention. In this report, we describe and interpret a comprehensive analysis of progress towards hepatitis A control and prevention through 2019 from the perspective of China’s National Immunization Program.
HTML
-
Domestically-developed live-attenuated and inactivated HepA vaccines were licensed in China in 1992 and 2002. Annual production of HepA vaccines increased steadily; between 1992 and 2007, 156 million doses of HepA were distributed, mainly for vaccinating school-aged children (4). Before 2008, all HepA vaccines were non-program vaccines, paid for out-of-pocket by families. Vaccination coverage was relatively low and varied geographically. Coverage was around 16% to 21% among those born before 2000 but was growing fast, reaching 53.8% in western provincial-level administrative divisions (PLADs) and 80.2% in eastern PLADs among the 2000–2007 birth cohorts.
In 2008, live and inactivated HepA vaccines were both integrated into the Expanded Program on Immunization (EPI). PLADs could select which vaccine to use, and most chose the live-attenuated HepA vaccine with its one-dose schedule at 18 months. A few PLADs selected the inactivated HepA vaccine with its two-dose schedule at 18 and 24 months. Nationally, coverage increased rapidly, reaching 88.1%–94.9% in all 3 regions of China among the 2008–2012 birth cohorts (Figure 1)(5).
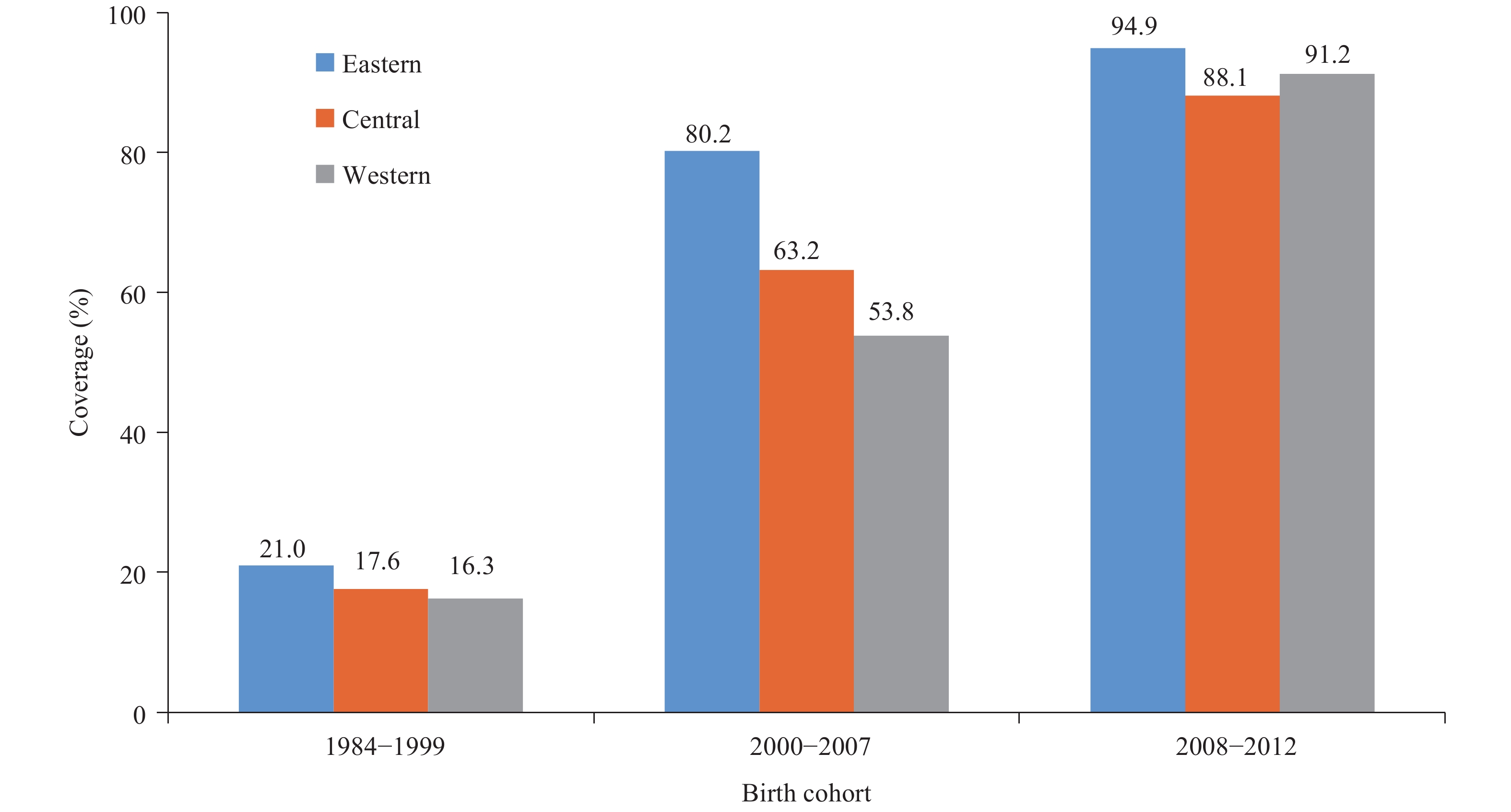 Figure 1.
Figure 1.Coverage of HepA vaccine by birth cohort and region, China (data from a national serological survey conducted in 2014).
Successful immunization programs ensure uniformly high HepA vaccine coverage, regardless of regional socioeconomic development. High coverage following HepA introduction into EPI was seen in other countries such as the United States and Israel (6-7).
-
In a 2006 serological survey, anti-HAV seroprevalence was shown to be increasing with age and was relatively low among children (8). Seroprevalence among children aged 2−14 years was significantly higher in the more developed eastern PLADs than in the developing central and western PLADs, which was consistent with varying vaccine coverage by regions (8-9).
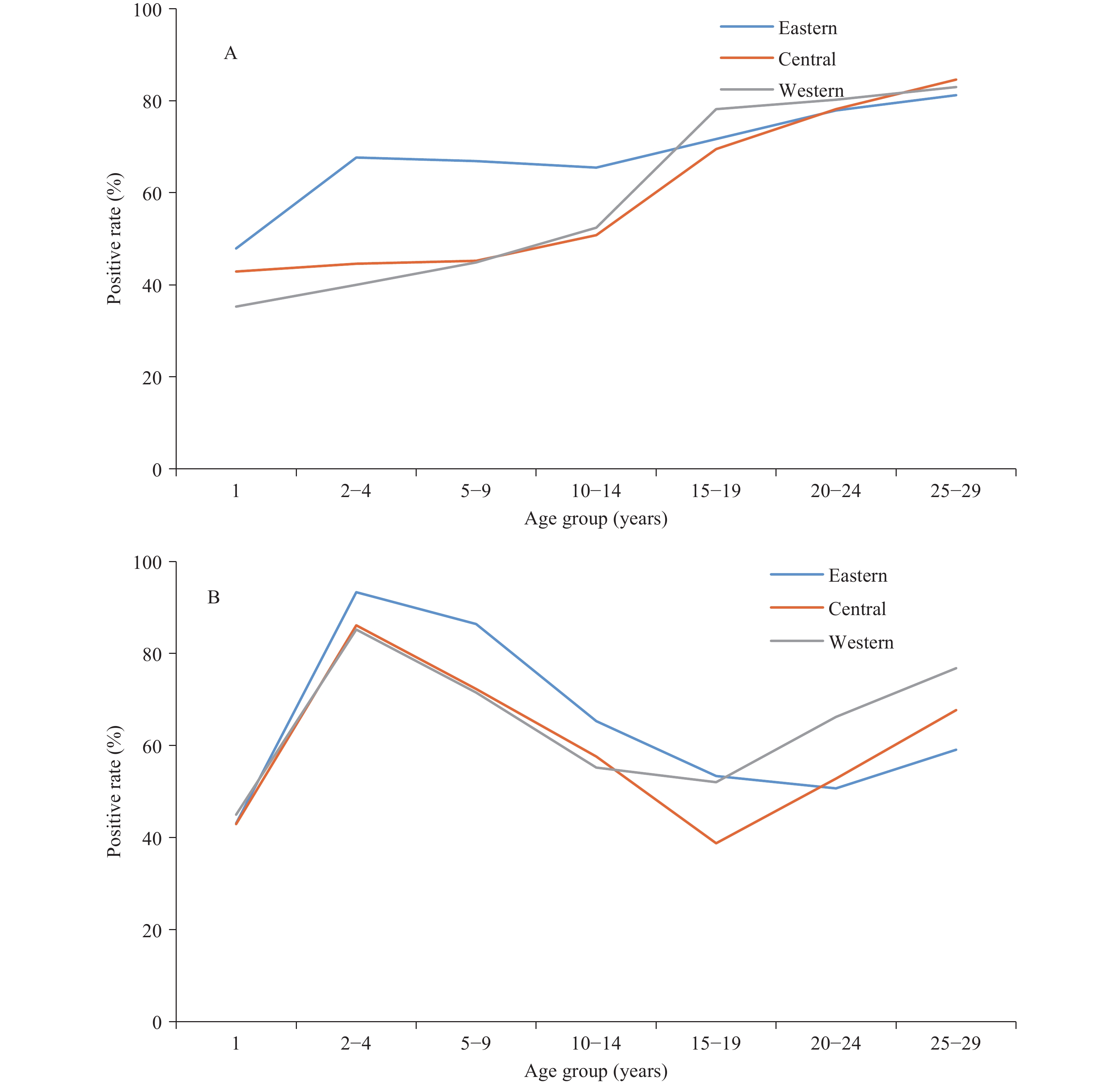
In 2014, after HepA vaccines had been included in the national program in all 3 regions for 6 years, a serological survey showed that anti-HAV seroprevalence among children aged 2−4 years was much higher and had little regional variation (Figure 2). However, seroprevalence was lower among individuals aged 15−19 years than other age groups, indicating an immunity gap among individuals aged 15−19 years. This immunity gap has been described in two other studies (10-11) and is a result of a rapid decrease in hepatitis A incidence following national HepA vaccine introduction causing a decrease in HAV exposure to those born prior to HepA vaccine introduction. Many in this age group, thus, escaped natural infection due to decreased circulation of HAV but also had not been immunized by HepA vaccine, which had low coverage before inclusion into EPI.
-
Hepatitis A incidence is closely related to both sanitation and vaccination. In China and abroad, declines in the incidence of hepatitis A have been well documented among vaccine-targeted populations, but incidence has also been shown to decline among non-vaccine-targeted populations due to indirect protection, e.g. through herd immunity (6-7,9). With better sanitation and widespread use of HepA vaccines in China, HAV-infection-induced immunity has been replaced by vaccine-induced immunity (8). Comparing 2004−2007 (pre-EPI) with 2008−2011, hepatitis A incidence decreased in all age groups and decreased further from 2012−2019. Incidence declines were seen in all PLADs, irrespective of region. In the pre-EPI era, hepatitis A incidence peaked at 5−9 years of age, but the peak was nearly eliminated after nationwide HepA vaccine introduction. Incidence declined in all age groups and in all three phases of EPI, and geographic disparities were greatly narrowed (9). (Figure 3).
 Figure 3.
Figure 3.Incidence of hepatitis A in three regions in different phases of the expanded program on immunization (EPI). (A) eastern region; (B) central region; (C) western region.
However, the hepatitis A incidence was high in the western PLADs, up to 4.28/100,000 among children under 5 years, although there were decreases in incidence. Possible factors for the higher incidence include lower EPI performance, less on-time vaccination, failure to vaccinate, and perhaps weaker sanitation in the western PLADs (12).
-
Study strengths were that the vaccination status of children aged under 15 years was obtained from official vaccination records; there has been long-term, consistent hepatitis A surveillance; and seroprevalence was determined by nationwide serological surveys with national-level laboratory testing. Limitations were that the vaccination status of individuals aged 15–29 years was based on recall; the passive hepatitis A surveillance system underreports mild cases or infections among individuals who did not seek medical attention; and that laboratory tests cannot distinguish between immunity from disease and immunity from vaccination.
-
The nationwide introduction of the HepA vaccination has eliminated most age and geographic disparities of hepatitis A disease. HepA vaccine introduction into the EPI system reduced disparities in coverage and seroprevalence in all three regions of China.
An immunity gap among older children and young adults indicated a potential risk of outbreaks among individuals who were born in the years immediately prior to nationwide HepA vaccine introduction. A catch-up vaccination effort could close this immunity gap and should be given serious consideration. An unexpectedly high hepatitis A incidence among children aged 0–4 years in the western PLADs should be closely monitored. Careful evaluation of the western PLADs’ immunization programs should be conducted to identify weakness in program performance and means to strengthen the programs.
Maintaining high vaccination coverage among children and strengthening western provincial-level programs will help ensure long-term progress towards elimination of hepatitis A in China.
Acknowledgement: The authors are grateful to the working staff in all levels of CDCs and hospitals that contributed to the prevention and control of hepatitis A in China. Thanks very much to Dr. Lance Rodewald for polishing language.
Conflict of interests: The authors declare no competing interests.
Fundings: The study was funded by the Project of Strengthening National Laboratories, Surveillance & Program Monitoring Systems, and Service Delivery Models for Viral Hepatitis in China.
| Citation: |



 Download:
Download: