-
Introduction: Aedes surveillance plays an important role in the risk warning and control of dengue in China. This study analyzed Aedes larval density and its indicated dengue risk in 23 provincial-level administrative divisions (PLADs) from 2016 to 2019 to provide scientific evidence for sustainable dengue management (SDM) in the future.
Methods: The Breteau index (BI) and Mosq-ovitrap index (MOI) methods were utilized for Aedes larvae surveillance in 23 PLADs with 3 categories based on dengue risk stratification and Aedes distribution. BI and MOI were calculated and then dengue risk warning was carried out for the guidance of SDM in these PLADs based on the findings of Aedes surveillance.
Results: The seasonal fluctuations of Aedes larval density were similar in six class I PLADs during 2016–2019, and the density of Aedes larvae was relatively high in the PLADs of Zhejiang, Hainan, and Fujian while it was relatively low in Yunnan and Guangdong. Except Shanghai and Jiangsu, the BI of all other class II PLADs had reached the dengue transmission threshold during the study period. In class III PLADs, the BI had reached dengue transmission threshold in most months in Shandong and Hebei during the study period. The MOI was higher than 5 from June to September in all the studied years in Guangdong and was higher in most studied years in Hunan. The MOI of Beijing in August reached dengue transmission or outbreak threshold from 2017 to 2019.
Conclusions and Implications for Public Health Practice: Most class I and class II PLADs were at risk of dengue transmission during the surveillance months, 2016–2019. Precise Aedes surveillance and risk warnings should be carried out in specific PLADs so as to provide scientific basis for SDM in the future.
-
In recent years, Aedes-borne diseases such as dengue (1), Zika, chikungunya, and yellow fever pose a serious public health threat. Globally, two species of Aedes mosquitoes, Aedes aegypti (L.) and Ae. albopictus (Skuse), play an important role for the transmission of diseases mentioned above. Under the impact of some natural and social factors such as climate and environmental change (2), urbanization (3), and globalization (4), etc., the distribution areas of Ae. aegypti and Ae. albopictus in China expanded in recent years (5–6), which has created a serious challenge to public health and the well-being of Chinese populations.
In order to meet the above challenges effectively, the theory of sustainable vector management (SVM) was proposed in 2004 (7) and was then continuously detailed and applied in Aedes-borne diseases control. Since the beginning of 2015, the central government transfer payment project of dengue has supported a systematic surveillance of Aedes mosquitoes, providing a basis for the control of Aedes-borne diseases in China. This paper introduces the progress of Aedes surveillance and its indicated risk based on surveillance data from 2016 to 2019. The findings will lay a solid foundation for sustainable dengue control in the future.
-
According to the guidelines for dengue prevention and control (2014), 23 surveillance PLADs were classified into 3 categories according to dengue risk stratification and Aedes distribution in China.
Class I areas included six PLADs (Guangdong, Yunnan, Guangxi, Hainan, Fujian, and Zhejiang) with several dengue outbreaks during the past years; class II areas were ten PLADs (Shanghai, Chongqing, Jiangsu, Anhui, Jiangxi, Henan, Hubei, Hunan, Sichuan, and Guizhou) with reported indigenous dengue cases and/or relatively high dengue risk; class III areas were seven PLADs (Beijing, Hebei, Shanxi, Tianjin, Shandong, Shaanxi, and Liaoning) with imported dengue cases reported and evidence of Aedes distribution.
For the density index of Aedes larvae surveillance, Breteau index (BI) and Mosq-ovitrap index (MOI) were utilized in this study where BI=(the number of positive containers or other water bodies per houses inspected)×100, while MOI=(positive ovitrap against the total number of effective ovitraps inspection)×100.
Surveillance was carried out twice a month with an interval of 10–15 days during mosquito activity season in Class I areas. It was conducted once a month from May to October in Class II areas, and from June to September in Class III areas.
According to the results of Aedes surveillance, dengue risk levels were estimated as the following: when BI<5 and/or MOI<5, dengue transmission is negated (no risk); when 5≤BI<10 and/or 5≤MOI<10, sporadic dengue can occur (low risk); when 10≤BI<20 and/or 10≤MOI<20, dengue outbreak may take place (medium risk); when BI≥20 and/or MOI≥20, dengue epidemic is imminent (high risk).
When BI≥5 and/or MOI≥5, a control plan for Aedes was formulated and sustainable Aedes management (SAM) measures were carried out, including targeting mosquito prevention, management of breeding sites, and adulticiding if necessary, to reduce the Aedes density below the dengue transmission threshold (BI<5 and/or MOI<5) so as to minimize dengue outbreak risk and achieve the SDM. It was worth noting that Aedes surveillance, control efficacy evaluation, and supervision were carried out during the process of SAM.
-
During 2016–2019, Aedes surveillance was carried out in approximately 436 counties and regions from 23 PLADs in China every year.
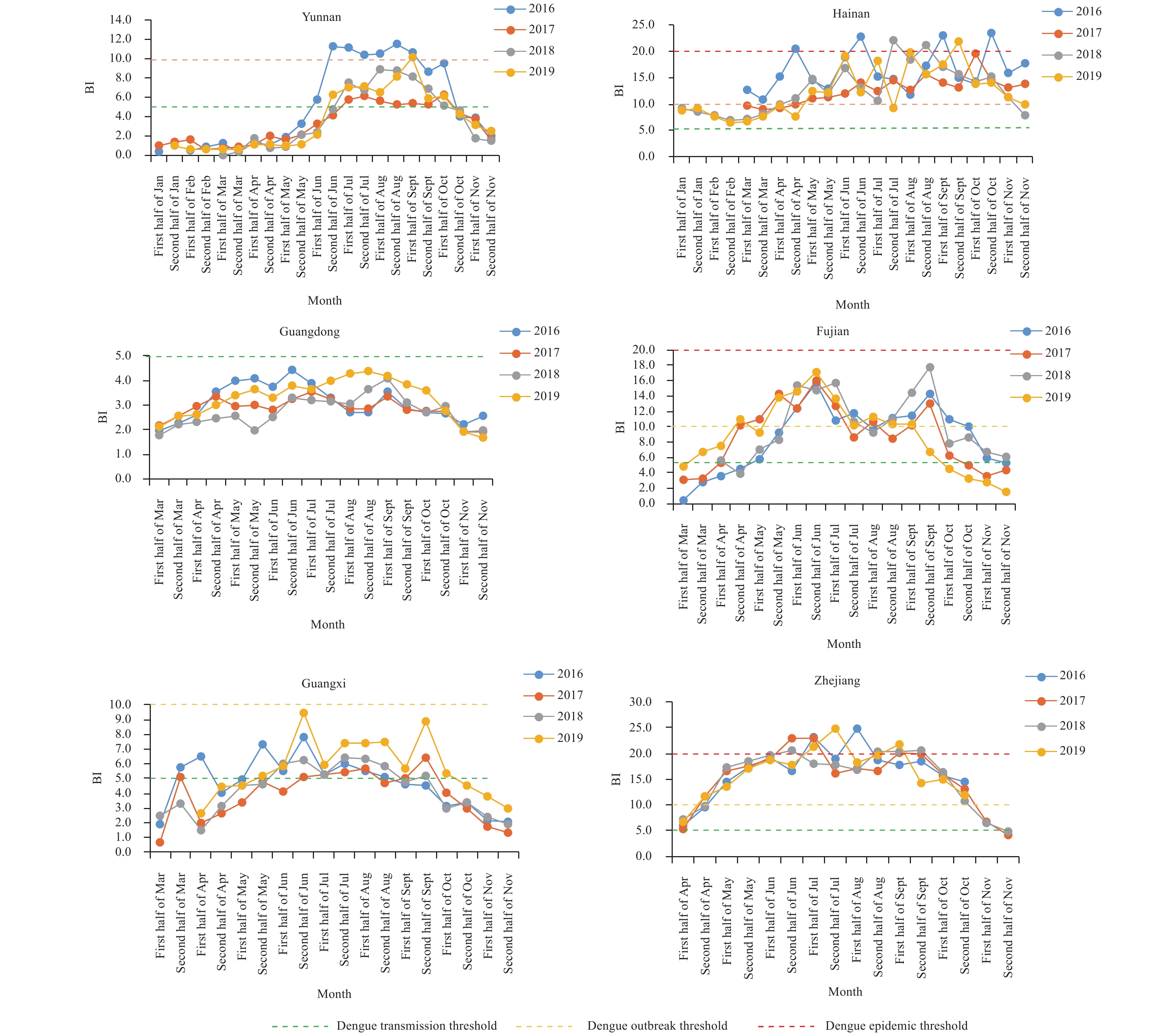
When comparing the BI among the Class I PLADs, similar seasonal fluctuations of Aedes larvae were observed in all PLADs from 2016–2019. In Yunnan, the BI exceeded 5 from the second half of June to the first half of October in 2016 and 2019. For Hainan, the BI exceeded 5 in all years and the BI were between 5 and 20 sporadically throughout the year. Concerning Fujian, the BI exceeded 5 from the first half of March to the second half of November and were higher than 10 from the second half of April to second half of September in 2017 and 2019. For Guangxi, the BI were higher than 5 from the second half of May to the first half of October in 2019. In Zhejiang, the BI exceeded 5 from the second half of April to the first half of November in all surveillance years.
However, all BI in Guangdong did not exceed 5 from 2016 to 2019. The risk indicated by BI surveillance was inconsistent with the actual dengue outbreak in Guangdong (Figure 1).
Excluding May and October in Shanghai and May, June, and October in Jiangsu, the BI in all Class II PLADs during the study months from 2016–2019 were higher than 5. Except Shanghai and Jiangsu, all other PLADs were at the risk of dengue outbreak or epidemic (Table 1).
Province Month BI Risk range* 2016 2017 2018 2019 Shanghai May 2.62 4.51 2.92 2.81 No risk June 4.95 5.65 6.01 3.93 No–Low risk July 6.18 7.58 7.32 6.38 Low risk August 5.49 7.76 7.12 4.97 No–Low risk September 4.66 6.79 6.09 3.93 No–Low risk October 3.23 4.96 2.47 2.45 No risk Chongqing May 6.25 5.99 7.44 5.78 Low risk June 11.10 9.45 12.30 9.23 Low–Medium risk July 10.34 12.33 11.64 10.68 Medium risk August 9.80 9.45 9.40 8.39 Low risk September 7.73 8.54 8.85 13.55 Low–Medium risk October 5.95 5.09 5.05 8.35 Low risk Jiangsu May 2.40 1.27 3.07 2.50 No risk June 4.86 3.43 3.45 2.92 No risk July 5.99 4.66 3.07 4.92 No–Low risk August 7.13 4.74 3.64 4.07 No–Low risk September 4.78 5.00 2.89 3.47 No–Low risk October 3.99 1.94 1.21 1.68 No risk Anhui May 3.82 5.11 7.48 4.75 No–Low risk June 9.06 8.31 11.27 7.59 Low–Medium risk July 14.60 14.99 14.24 12.06 Medium risk August 11.96 18.14 14.75 12.24 Medium risk September 9.57 16.69 11.67 11.69 Low–Medium risk October 5.70 6.85 6.63 5.00 Low risk Jiangxi May 14.10 0.95 17.50 15.38 No–Low risk June 23.60 15.90 13.56 14.34 Medium–High risk July 12.48 7.90 11.00 11.38 Low–Medium risk August 12.67 9.62 7.55 10.56 Low–Medium risk September 8.76 8.89 8.00 7.69 Low risk October 15.33 4.63 6.70 2.86 No–Medium risk Henan May 10.10 14.70 10.50 4.69 No–Medium risk June 21.39 17.36 16.06 11.57 Medium–High risk July 31.88 26.22 24.90 22.92 High risk August 32.40 31.91 23.01 26.38 High risk September 32.07 23.62 13.49 18.91 Medium–High risk October 14.41 13.09 9.50 9.68 Low–Medium risk Hubei May 10.90 7.11 7.06 6.21 Low–Medium risk June 13.42 10.83 7.83 8.08 Low–Medium risk July 19.83 13.54 8.22 8.03 Low–Medium risk August 13.76 9.85 5.88 10.22 Low–Medium risk September 8.64 17.98 7.54 3.99 No–Medium risk October 7.22 5.87 3.53 7.14 No–Low risk Hunan May 17.90 6.90 13.93 12.60 Low–Medium risk June 17.90 31.20 13.83 26.47 Medium–High risk July 9.65 12.20 13.09 26.53 Medium–High risk August 10.95 4.14 13.23 16.48 No–Medium risk September 10.95 12.61 11.53 13.91 Medium risk October 3.44 5.55 9.78 4.98 No–Low risk Sichuan May 4.90 10.15 12.24 9.14 No–Medium risk June 7.60 15.39 11.95 12.89 Low–Medium risk July 8.33 18.23 15.96 28.31 Low–High risk August 13.36 12.62 14.02 11.64 Medium risk September 7.56 6.87 7.75 11.48 Low–Medium risk October 8.98 3.26 3.78 8.57 No–Low risk * No risk (BI<5): dengue transmission negated; Low risk (5≤BI<10): sporadic dengue occurrence; Medium risk (10≤BI<20): dengue outbreak risk; High risk (BI≥20): dengue epidemic risk. Table 1. Breteau index (BI) in class II areas in China (2016–2019).
As for the BI in Class III PLADs, the BI exceeded 5 in Hebei while it did not exceed 5 in Liaoning and Tianjin during the period of surveillance from 2016 to 2019. The BI exceeded 5 in most months in Shandong and Shaanxi and for a few months in Shanxi (Figure 2).
The MOI was another key index that was utilized by some PLADs for the surveillance of Aedes larvae. This indicator was more sensitive than BI in some PLADs, especially in Guangdong. The MOI exceeded 5 from the first half of May to the first half of October in Guangdong and from the second half of May to the second half of September in Guangxi for almost all study years (Figure 3).
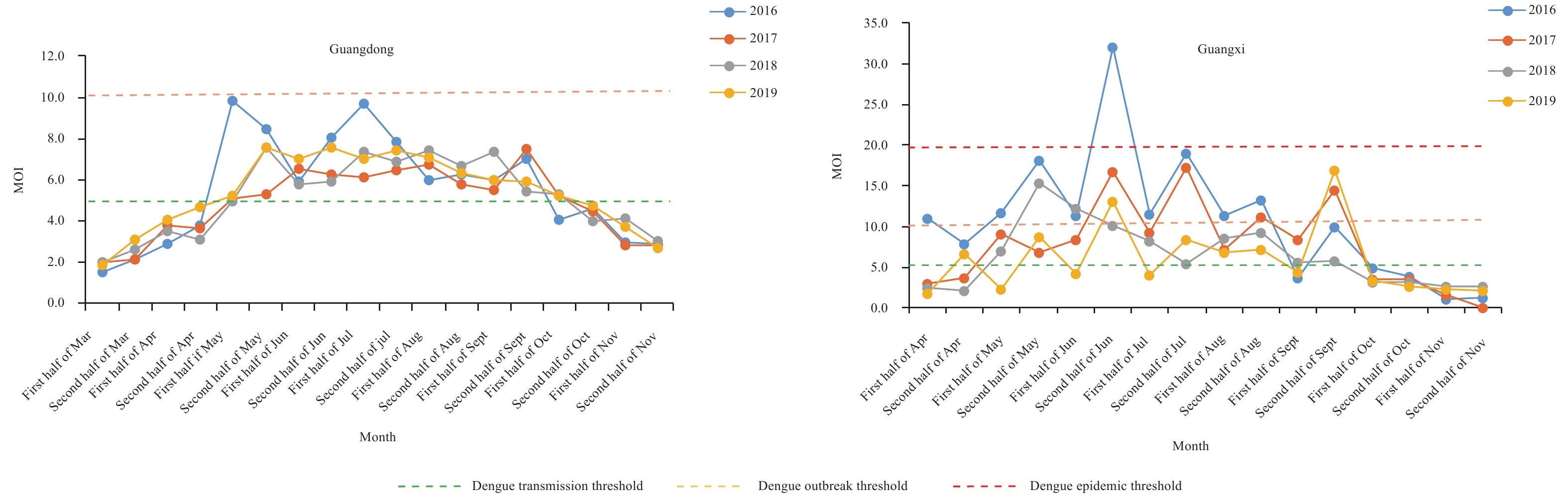 Figure 3.
Figure 3.MOI of Guangdong and Guangxi in Class I areas of China (2016–2019). BI=breteau index; MOI=mosq-ovitrap index.
For Guizhou, the MOI exceeded 5 from June to September in all studied years and exceeded 5 in May and October in 2016. In Hunan, the MOI exceeded 5 from June to September in most study years. Beijing, as a representative of Class III area, had a relatively high MOI in August and was at the risk level between dengue transmission to epidemic risk, especially from 2017 to 2019 (Table 2).
Class Province Month MOI Risk level* 2016 2017 2018 2019 II Guizhou May 5.29 4.61 4.71 4.13 No–Low risk June 7.81 7.59 6.73 5.45 Low risk July 8.87 7.43 9.28 8.11 Low risk August 11.03 5.75 9.01 10.08 Low–Medium risk September 9.05 5.42 5.84 7.86 Low risk October 5.74 4.09 3.96 4.34 No–Low risk Hunan May 3.12 1.35 1.44 0.78 No June 9.83 3.77 2.28 5.11 No–Low risk July 8.89 5.59 5.18 6.04 Low risk August 6.49 5.18 3.40 6.06 No–Low risk September 6.49 6.72 3.81 4.49 No–Low risk October 3.52 3.76 0.86 1.58 No III Beijing June 3.74 2.34 1.27 1.06 No July 3.57 7.45 3.99 3.05 No August 4.87 10.76 8.71 9.30 No–Medium risk September 5.87 3.69 4.86 1.16 No–Low risk * No risk (MOI<5), dengue transmission negated; Low risk (5≤MOI<10), sporadic dengue occurrence; Medium risk (10≤MOI<20), dengue outbreak risk; High risk (MOI≥20), dengue epidemic risk. Table 2. Mosq-ovitrap index (MOI) in class II and III areas in China (2016–2019).
-
In this study, we focused on the findings of Aedes larval surveillance in China during 2016–2019. The findings are the basis of risk warning and strategic control of dengue.
In Zhejiang and Hainan, the BI exceeded the threshold of dengue transmission in nearly all surveillance months and the threshold of dengue outbreak in some surveillance months during 2016–2019. Dengue outbreaks occurred frequently in Zhejiang during recent years and in Hainan in 2019. High density of Aedes larvae may be one of the driving factors of dengue outbreak in these two PLADs during recent years. Aedes larval density in Fujian and Guangxi was also high and could be attributed to the increased incidence, frequent local outbreaks, and expansion of dengue distribution in PLADs during recent years (8).
With long border lines, large amount of cross-border population movement, and a suitable climate and ecological environment for Aedes mosquitoes, indigenous dengue has occurred frequently in Yunnan since 2013. Dengue has an external incubation period of usually 8 to 10 days and an internal incubation period of about 5 to 8 days. The fact was that sporadic dengue cases or dengue outbreaks often occurred in Yunnan from the first half of July to the first half of November. Therefore, the possible outbreak period of dengue as indicated by combining periods of high Aedes density plus incubation periods was basically consistent with the real periods of emergence of local sporadic cases or outbreak in Yunnan (9). As a result, the local government organized intense mosquito control efforts and had a lower average BI than other class I PLADs during 2016–2019.
The MOI was another core larval density index and seemed to be more suitable for dengue risk warnings in Guangdong. In recent years, we have observed dengue outbreaks in many cities of Guangdong with BI less than 5 during 2016–2019, but dengue has occurred continuously in Guangdong since 2014 (10). Single use of BI could not assess the risk of dengue comprehensively and objectively possibly due major cities with large floating populations decreasing the sensitivity of BI. Based on previous interviews with staffs of local CDCs, the difficulty of household entry in Guangdong may lead to low household access, which in turn decreased the sensitivity of BI surveillance for dengue risk assessment.
The other Aedes surveillance tools with similar catching mechanism but different configurations, such as CDC autocidal gravid ovitraps, larvitraps, Mosq-ovitraps (China), etc. have been implemented widely around the world (11). In this study, Mosq-ovitrap was utilized and the MOI was calculated accordingly. The risk as indicated by average MOI in Guangdong reached dengue transmission risk from the first half of May to the first half of October for almost all studied years. One of the advantages of MOI surveillance is that the trapping does not have to be conducted in households. In the future, the Mosq-ovitrap method should also be carried out in some PLADs with potential risk of dengue transmission or outbreak despite low BI to augment the deficiencies of BI surveillance.
This study demonstrated that the Aedes larval density was always low in Shanghai and Jiangsu, and this may be the main reason why there was no local outbreak of dengue in these two PLADs during recent years. However, Chongqing and Jiangxi witnessed the first outbreak of indigenous dengue in 2019 and these two PLADs had high BI in the same year. And BI of Henan and Anhui were also high and experienced local dengue transmission or outbreaks during recent years.
As for BI surveillance in Class III PLADs, we need to emphasize the transmission risk of dengue in Hebei due to the high density of Aedes larvae during recent years. Furthermore, the BI exceeded 5 in most months in Shandong, a PLAD with two recent dengue outbreaks. In Beijing, the MOI in August reached dengue transmission risk level during 2017–2019. Once an imported dengue case occurs, the possibility of local dengue transmission or outbreak appears.
Due to the deficiencies in the local capabilities of Aedes surveillance and availability of surveillance data, this study calculated the overall average density of Aedes species rather than calculating the density of Ae. aegypti and Ae. albopictus separately. In addition, a dengue outbreak is driven by multidimensional factors and there are a certain limitations to using a single vector index for dengue risk assessment. Therefore, further studies are warranted to distinguish Ae. aegypti from Ae. albopictus for precise surveillance of Aedes and for carrying out dengue risk warnings using multidimensional factors to provide scientific basis for SDM in the future.
Acknowledgments: We thank all research staff from local Centers for Disease Control and Prevention for collection of data.
Funding: This study was supported by the National Natural Science Foundation of China (No. 81703280) and National Science and Technology Major Project of China (No. 2017ZX10303404005001).
HTML
| Citation: |



 Download:
Download:





