-
Cervical cancer is the fourth most common cancer among women worldwide (1). Due to immunosuppression caused by human immunodeficiency virus (HIV) infection and the prolonged life expectancy associated with antiretroviral treatment (ART), cervical cancer has been one of the most common malignancies among HIV-positive women (2), and the World Health Organization (WHO) has recommended providing HIV-positive women with cervical cancer prevention and screening programs (3). An estimated 158,600 women in China are infected with HIV, but there is a lack of evidence to support the development of strategies for cervical cancer prevention and control that target HIV-positive women in China (4). Using data from a prospective study, we found that the detection rate of cervical intraepithelial neoplasia (CIN) grades 2 or worse (CIN2+) among HIV-positive women was 4.1% at baseline, and the incidence of CIN2+ was 0.1% in our follow-up survey. High-risk human papillomavirus (hrHPV) positive and early (<18 years old) sexual debut was associated with CIN2+ detection. The results draw attention to the need for regular cervical cancer screening among HIV-positive women and provides evidence on which to develop a cervical cancer screening program in China.
The data was derived from a prospective study on the co-infection of human papillomavirus (HPV) among HIV-positive women. The HIV-positive women were offered screening with an HPV test, cytology, and biopsy at baseline in 2015. The follow-up survey followed the same procedure as the baseline survey, which was conducted 1.5 years later on average
 . HIV-positive women aged 18–49 years were recruited from randomly selected townships in 5 counties of 3 high HIV-burden provincial-level administrative divisions (PLADs) including Yunnan, Guangxi, and Xinjiang. Eligible participants were those with no debilitating illness who had no previous history of cervical neoplasia or uterectomy. All participants provided informed consent to participate in the study. Ethical approval was obtained from the Institutional Review Board of the National Center for Women and Children’s Health, China CDC (No. FY2015–014).
. HIV-positive women aged 18–49 years were recruited from randomly selected townships in 5 counties of 3 high HIV-burden provincial-level administrative divisions (PLADs) including Yunnan, Guangxi, and Xinjiang. Eligible participants were those with no debilitating illness who had no previous history of cervical neoplasia or uterectomy. All participants provided informed consent to participate in the study. Ethical approval was obtained from the Institutional Review Board of the National Center for Women and Children’s Health, China CDC (No. FY2015–014).A questionnaire survey was used to collect sociodemographic, reproductive, and sexual behavioral information. The medical records were reviewed to extract data on ART and laboratory results. Trained gynecologists provided gynecological physical examinations and collected cytological specimens. The cytological specimens were sent to a designated laboratory for cytological examination and HPV DNA test with the Cobas 4800 HPV test [Roche Molecular Diagnostics (Shanghai) Co. Ltd., Shanghai, China] to produce results for HPV16 and HPV18, and a pooled result on the other 12 hrHPV types (31, 33, 35, 39, 45, 51, 52, 56, 58, 59, 66, and 68b). In the baseline survey, another cytological specimen was collected and processed for 14 hrHPV types (16, 18, and the other 12 types).
HIV-positive women with cytology results of atypical squamous cells of undetermined significance (ASCUS) or worse (≥ASCUS) or hrHPV positive received colposcopy. The participants with abnormal colposcopy findings underwent biopsies, and those diagnosed with CIN2+ were referred for proper management. HIV-positive women with negative hrHPV and negative cytology or with negative colposcopy were deemed negative.
The blood samples were collected and tested for syphilis and hepatitis B surface antigen (HBsAg). The results of the CD4 lymphocyte count tests and HIV viral load tests within six months of when the fieldwork was conducted were either obtained from clinical records or from laboratory tests at the sites.
The analysis is based on women diagnosed with CIN2+ and those who had negative results from cervical screening in both baseline and follow-up surveys. The data was analyzed using SPSS software (version 23.0, IBM Corp, Armonk, NY, USA). We presented categorized variables as frequencies and proportions and used Pearson’s chi-square test and Fisher’s exact test as appropriate. Factors with a p-value of <0.1 were entered into a binary logistic regression to build the final model. A p-value of <0.05 was considered statistically significant and calculated for adjusted odds ratios (AOR) and 95% confidence intervals (95%CI).
The recruitment, screening, and diagnosistic processes are shown in Figure 1. During the baseline survey, 695 HIV-positive women were recruited, and 617 (88.8%) women completed the screening procedure. Of these, 25 women [4.1% (95%CI: 2.5%–5.6%) of those screened] had CIN2+. In the follow-up survey, among the 670 women who followed up, 462 (69.0%) completed the screening procedure, of whom two cases of CIN2+ [0.4% (95%CI: 0%–1.0%)] were detected. One of the two women (CIN3) was diagnosed with HPV58 infection, ASCUS, and CIN1, and another woman (CIN2) was diagnosed with HPV33/68 infection and negative cytology and colposcopy at the baseline. No cervical cancer was detected. The data of 27 women with CIN2+ and 442 women with negative results in both baseline and follow-up surveys were analyzed.
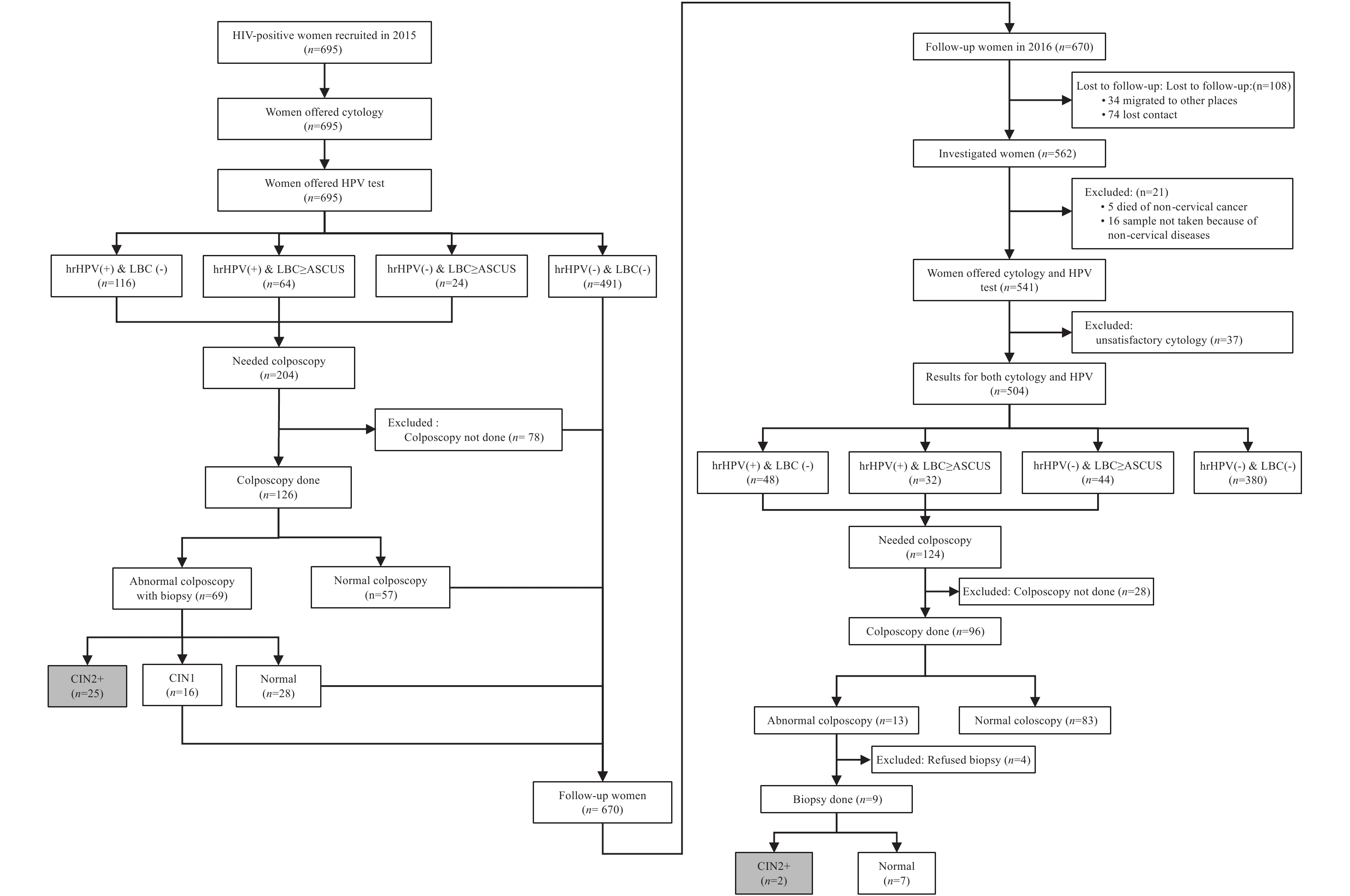 Figure 1.
Figure 1.Cervical cancer screening and diagnosistic processes of the baseline and follow-up surveys among HIV-positive women from HIV high-burden areas in China, 2015–2016. Abbreviation: LBC=liquid-based cytology; hrHPV=high-risk human papillomavirus; HIV=human immunodeficiency virus; CIN=cervical intraepithelial neoplasia.
We present the univariate and multivariate analysis of sociodemographic characteristics, biological indicators of HIV infection, and STI coinfection between CIN2+ and negative women in Table 1. The distributions of age at sexual debut, hrHPV infection, and syphilis differed significantly between those two groups. Compared with women without hrHPV infection, those with single or multiple hrHPV infection or women with HPV16/18 or other hrHPV infection were more likely to have CIN2+ (trend χ2=81.84, p<0.001, trend χ2=30.69, p<0.001, respectively) (Figure 2). After controlling for age, we found hrHPV infection and early (<18 years old) sexual debut were associated with the occurrence of CIN2+ (AOR=49.1, 95%CI: 11.14–216.1, p<0.001; AOR=3.4, 95%CI: 1.2–10.2, p=0.03).
Characteristics No. of observations CIN2+ Normal Univariate analysis Multivariate analysis n (%) n (%) n (%) χ2 p-value AOR (95%CI) p-value Total 469 27 442 PLAD 1.68 0.43 Yunnan 196 (41.8) 10 (37.0) 186 (42.1) Guangxi 149 (31.8) 7 (26.0) 142 (32.1) Xinjiang 124 (26.4) 10 (37.0) 114 (25.8) Age (years old) 0.19 0.91 18–29 79 (16.8) 4 (14.8) 75 (17.0) 1 30–39 277 (59.1) 17 (63.0) 260 (58.8) 2.3 (0.6–9.5) 0.23 40–49 113 (24.1) 6 (22.2) 107 (24.2) 2.8 (0.6–14.1) 0.22 Ethnic group 4.11 0.13 Han and others 210 (44.8) 12 (44.4) 198 (44.8) Uighur 108 (23.0) 10 (37.1) 98 (22.2) Jingpo/Dai 151 (32.2) 5 (18.5) 146 (33.0) Education level 0.004 0.95 Primary school and below 223 (47.5) 13 (48.1) 210 (47.5) Junior high and above 246 (52.5) 14 (51.9) 232 (52.5) Current employment 0.86 0.65 Farmer 216 (46.1) 11 (40.7) 205 (46.4) Housewife 153 (32.6) 11 (40.7) 142 (32.1) Other 100 (21.3) 5 (18.6) 95 (21.5) Marital status 0.44 0.51 Married/cohabiting 355 (75.7) 19 (70.4) 336 (76.0) Single/divorced/widowed 114 (24.3) 8 (29.6) 106 (24.0) Annual income (RMB per capita) 0.10 0.75 ≥5,000 335 (71.4) 20 (74.1) 315 (71.3) <5,000 134 (28.6) 7 (25.9) 127 (28.7) Residence registration 1.80 0.18 Urban 107 (22.8) 9 (33.3) 98 (22.2) Rural 362 (77.2) 18 (66.7) 344 (77.8) Age at sexual debut* 0.003* Early (<18 years old) 63 (13.6) 9 (36.0) 54 (12.3) 3.4 (1.2–10.2) 0.03 Late (≥18 years old) 400 (86.4) 16 (64.0) 384 (87.7) 1 Lifetime sexual partners 0.90 0.34 1 215 (45.8) 10 (37.0) 205 (46.4) ≥2 254 (54.2) 17 (63.0) 237 (53.6) Currently smoking 1.00* Yes 19 (4.1) 1 (3.7) 18 (4.1) No 450 (95.9) 26 (96.3) 424 (95.9) Gravidity 0 1.00 0–3 330 (70.4) 19 (70.4) 311 (70.4) 4–9 139 (29.6) 8 (29.6) 131 (29.6) Parity 0.58* 0–2 398 (84.9) 22 (81.5) 376 (85.1) 3–6 71 (15.1) 5 (18.5) 66 (14.9) HIV infection route 0.39* Sexual contact 442 (94.2) 27 (100.0) 415 (93.9) Others 27 (5.8) 0 (0.0) 27 (6.1) CD4 lymphocyte count (/mm3) 0.36 0.55 <350 100 (21.3) 7 (25.9) 93 (21.0) ≥350 369 (78.7) 20 (74.1) 349 (79.0) HIV viral load (copies/mL) 0.52* <1,000 419 (89.3) 23 (85.2) 396 (89.6) ≥1,000 50 (10.7) 4 (14.8) 46 (10.4) ART 0.61* Yes 443 (96.3) 25 (100.0) 418 (96.1) No 17 (3.7) 0 (0.0) 17 (3.9) hrHPV infection 71.96 <0.001 Yes 106 (22.6) 24 (88.9) 82 (18.6) 49.1 (11.1–216.1) <0.001 No 363 (77.4) 3 (11.1) 360 (81.4) 1 Syphilis 0.03* Yes 22 (4.7) 4 (14.8) 18 (4.1) 4.1 (0.9–18.2) 0.07 No 446 (95.3) 23 (85.2) 423 (95.9) 1 HBsAg positive 1.00* Yes 32 (6.9) 1 (3.7) 31 (7.0) No 435 (93.1) 26 (96.3) 409 (93.0) Abbreviations: CIN2+=cervical Intraepithelial neoplasia grades 2 or worse; PLAD=provincial-level administrative division; hrHPV=high-risk human papillomavirus; AOR=adjusted odds ratio; 95%CI=95% confidence intervals. ART: antiretroviral treatment; HBsAg positive=hepatitis B surface antigen.
* Fisher’s exact test; Factors significant at p<0.1 in univariate analysis and age were entered into the regression model.Table 1. Characteristics of HIV-positive women with CIN2+ and with negative result of cervical screening in HIV high-burden areas in China, 2015–2016
 Figure 2.
Figure 2.The detection of CIN2+ associated with different hrHPV infection groups among HIV-positive women from HIV high-burden area in China, 2015–2016. (A) Chi-square and p-value for trend for multiple hrHPV, single hrHPV, and no hrHPV was trend χ2=81.84, p<0.001. (B) Chi-square and p-value for trend for HPV16/18, other hrHPV, and no hrHPVwas trend χ2=30.69, p<0.001. Abbreviations: hrHPV=high-risk human papillomavirus; HIV=human immunodeficiency virus; CIN2+=cervical Intraepithelial neoplasia grades 2 or worse; other hrHPV=HPV31, 33, 35, 39, 45, 51, 52, 56, 58, 59, 66, and 68b.
HTML
-
In this study, we found that the detection rate (4.1%) of CIN2+ among HIV-positive women at baseline was higher than that of general rural women aged 35−64 years (0.1%) in China (5). Previous research reported an accumulated 6-year risk of CIN2+ was 1.2% among rural women in Shanxi province (6), and the incidence of CIN2+ was 283.79 per 100,000 among women aged between 20 and 65 years old (7). Although newly detected cases were rare and more data is needed for further analysis, our findings show a relatively high incidence rate of CIN2+ among HIV-positive women in a shorter follow-up period compared with previous studies (6). Our results confirmed that HIV-positive women were a high-risk group for cervical precancerous lesions and suggested that the progress of CIN in HIV-positive women was much faster than that among HIV-negative women. The majority of our participants had low educational status and economic status and were from ethnic minority groups and rural areas. Women with those socioeconomic characteristics were significantly less likely to receive cervical cancer screening in China (8). Our findings indicated the needs of cervical cancer screening of HIV-positive women might not be satisfied.
HPV persistent infections are precursors of cervical cancer with 14 types classified as oncogenic (9). The proportion of hrHPV infection (22.6%) among HIV-positive women in this study was much higher than that among the general population of women (16.8%) (10). We confirmed that hrHPV infection among HIV-positive women was highly associated with precancerous lesions, and our findings also demonstrated that there was a higher risk related to multiple hrHPV infections and HPV16/18 infections, which might be due to the cumulative effect of multiple infections and the strong pathogenic impact of HPV16/18 (11). The Advisory Committee on Immunization Practice (ACIP) has recommended HPV vaccination among HIV-positive women to reduce the risk of cervical cancer in this group (12).
Early sexual debut, multiple sexual partners, smoking, early pregnancy, menopause, and lower educational level were found to be risk factors of cervical cancer in Chinese females (13). For HIV-positive women, lower CD4 lymphocyte count levels might relate to developing cervical cancer (14). However, in our study, except for hrHPV infection, we only found the association between early sexual debut and CIN2+, which might be because such sexual behavior enhanced the probability of exposure to HPV. HIV, HPV, and STIs could all be transmitted through sexual contract and were the main route of infection for women. Though we didn’t find a correlation between syphilis and CIN2+ in multivariate analysis, further research was needed. Our results implied the importance of behavioral change programs for HIV-positive women to promote safe sexual behavior.
This study was subject to a few limitations. The small sample size and the short period of follow-up limited our ability to detect women with cervical cancer. Our study sample also did not allow for generalization of results nation-wide. Future studies are needed to understand the detection and associated factors of cervical cancer among HIV-positive women with a larger sample in both low and high HIV-burden areas in China.
In conclusion, the study found a high detection rate of CIN2+ and the relatively faster progress of CIN among HIV-positive women from high HIV-burden areas of China, which reflected a higher risk of developing cervical cancer of HIV-positive women. Hence, HIV-positive women might benefit from regular cervical cancer screening with a shorter intervals between screenings. As hrHPV infection was associated with the detection of CIN2+, we recommend that cervical cancer screenings apply an approach that combines hrHPV tests and cytology if conditions permit. Our findings that early sexual debut was an associated factor of CIN2+ and points to the need for behavioral change program for HIV-positive women to promote safe sexual behavior.
Conflict of interest: No conflict of interest were reported.
Acknowledgments: We would like to express our thanks to staff from study sites who contributed to the fieldwork as well as all HIV-positive women who participated the research.
Funding: This study was supported by grants from the UNICEF China (IR-5.2 PMTCT and Pediatric Services, Activity No. 0860/A0/04/705/052/001).
| Citation: |



 Download:
Download:




