-
The emergence of multidrug-resistant tuberculosis (MDR-TB) was a major public health threat and challenge for the End TB Strategy proposed by the World Health Organization (WHO). The WHO reported 157,000 MDR-TB cases in 2020 worldwide (1). About 95% of identified MDR-TB patients were enrolled in treatment using second-line drug therapy; however, unfavorable treatment outcomes were high, as treatment success for MDR-TB is only 59.3%. These unfavorable outcomes include loss to follow-up (LTFU, 14.3%), death (13.3%), and treatment failure (9.1%). China in particular has seen a major challenge posed by limited treatment success (54%) and high loss to follow-up rate (31.9%) (1). A simulated mathematical model showed that, if TB response is maintained at the current level, the prevalence of MDR-TB will triple by 2050 (2).
A previous qualitative study showed that barriers to MDR-TB treatment success included the long treatment course, poor treatment adherence, catastrophic financial barriers to treatment, adverse effects to second-line drugs, and lack of support from family, doctors, and peers (3). Even further, without an electronic system that can integrate all key strategic information, it is very technically difficult to conduct timely monitoring of disease progression and manage patients’ follow-up on a long-term basis.
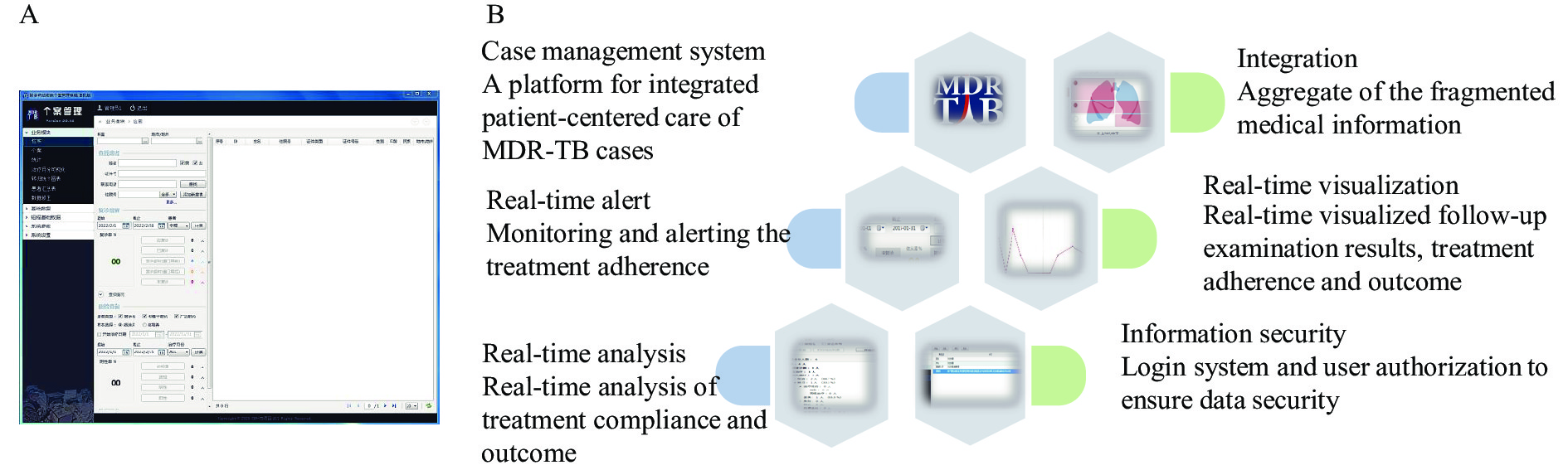
To overcome the barriers of MDR-TB treatment, the Yunnan CDC (YNCDC) collaborated with Family Health International (FHI 360) in 2017, under the technical guidance of the Tuberculosis Prevention and Control Center of the China CDC, to develop and implement the Control and Prevention of MDR-TB program (CAP-TB). The program introduced a patient-centered, Comprehensive Supportive Care (CSC) services model to strengthen MDR-TB care (4). Based on this CSC framework, the program developed an integrated, supportive information system for MDR-TB patient management: the MDR-TB Case Management System (CMS, Figure 1A). This study aims to introduce the CMS in pilot areas and evaluate whether the system helps improve treatment management of MDR-TB.
-
The Yunnan CDC and FHI 360 designed and developed the CMS under the guidance of the China CDC in 2017. The CMS was developed based on the Microsoft Visual Studio (version 12.00, Microsoft, Redmond, American). The required supporting software included the Microsoft. NET Framework (version 4.7.1, Microsoft, Redmond, American) and Microsoft Visual Studio (version 12.00, Microsoft, Redmond, American). The CMS was designed based on the concept of case management. An integrated, patient-centered system means dependable communication and coordination throughout the entire process of MDR-TB care, which best meets the healthcare needs of TB patients. The integrated system intensifies information aggregation and data visualization, and includes useful features ranging from warnings about adverse reactions and timely reminders for upcoming appointments to tracking patients throughout treatment (Figure 1B).
-
The integrated, patient-centered supportive information CMS was finalized in 2017.
It includes 5 key functionalities:
Case management: The CMS includes the functions of case listing, case filtering, case detailing, and case registration. It is used to collect the clinical data of diagnosed MDR-TB patients who have enrolled in treatment and provide continuous care throughout the entire treatment course. The CMS records demographic and socioeconomic background, diagnosis information, medical history, clinical indicators, treatment and supportive care records, as well as results from patient intake assessment tools — including mini nutritional assessments, social support instruments, and depression and anxiety tests.
Case information retrieval: The CMS can filter and search a registered case by one or multiple conditions. Case information aggregates longitudinal and horizontal data for the entire treatment course. The longitudinal data refers to the individual record across follow-up visits made by the patients. The horizontal data indicates clinical data of individual patients from various departments of the hospital, such as diagnosis and treatment for inpatients; clinical summary reports for outpatients, radiology, and chest X-rays; laboratory blood tests; and sputum smears and cultures.
Follow-up query and alert: Based on the treatment regimen and follow-up algorithm for MDR-TB care, the CMS can automatically generate a list of patients who are expected to attend treatment visits within a particular period of time, use queries to identify whether the patients have attended their scheduled visits on time or not, and track whether they have completed sputum tests and other required examinations for treatment monitoring. The CMS can also alert the case manager about patients who have failed to show up for their scheduled visits.
Real-time analysis and visualization: The CMS monitors critical indicators of adherence and treatment. The CMS also generates real-time statistical analysis and visualization of sputum test results, treatment adherence, and adverse reactions — either at individual or population levels. Even further, the CMS can automatically determine treatment outcomes according to the CMS status of patients and sputum tests performed.
Share and transfer CMS data: In order to make it easier for users to carry out further statistical analysis or share data with others, the system supports exporting, importing and printing of data.
The CMS was designed to monitor critical indicators of real-time regular follow-up rate (RFUR). In practice, a standard MDR-TB regimen is a treatment course of 18–20 months in accordance with the Chinese National Tuberculosis Program (CNTP). The regimen contains an intensive phase of 6 months and a continuous phase of 12–14 months. All MDR-TB patients are expected to pay for clinical visits for treatment monitoring (including sputum smear microscopy and cultures, chest X-rays, routine blood tests, and liver-kidney function tests) each month during the intensive phase and every two months in the continuous phase.
Regarding the population level, the RFUR in a fixed period t (normally one month) can be calculated with the following equation
$$ {RFUR}_{t}=\frac{{No.\;actual\;visits}_{t}}{{No.\;expected\;visits}_{t}} $$ (1) According to the individual treatment regimen, the expected visits is defined as the number of patients expected to visit during a particular period t (e.g., from May 1st, 2021 to May 31st, 2021). The actual visits is defined as the actual number of patients who have both visited the TB care facility and had monitoring treatment tests performed during the given period (e.g., May 2021).
The calculation of
$ {RFUR}_{t} $ is a dynamic process, depending on not only different periods of time t but also the change of the population cohort in the given period t, which refers to the new patients introduced into the treatment cohort or patients whose treatment outcomes occurred and left the cohort. The expected visits included the number of patients returning to visit on time (within one week before or after the expected date of visit), the patients coming ahead of schedule (before one week of the expected date of visit), the patients with delayed visits (after one week of the expected date of visit), and the patients who did not return to visit (within two weeks before or after the expected date of visit).The comparison of the features between the paper-based reports, the Tuberculosis Information Management System (TBIMS) and the CMS, is presented in Table 1.
Feature Paper-based reports TBIMS CMS Utilization Collected case and patient data for record Electronic report and surveillance of the TB disease in a large-scale population The supplementary of TBIMS to enhance the case management Content Paper-based MDR-TB case report Web-based MDR-TB case report and registration Integrated demographic, socioeconomic, epidemiological, clinical, treatment, treatment adherence, nutritional and psychological assessment; also available for 9-month short regimen for MDR-TB Data collection Collected data by paper-and pencil Collected data based on website Collected data by CMS client computer Data storage Stored data by paper-based records Data were automatically uploaded to data center Data were locally stored by SQL file Data transmission Mail or delivery Data transmitted by network service Data transmitted by packaged SQL file Real-time statistical analysis Unavailable Simple statistics on website Real-time statistical analysis in CMS client. CMS computed adherence rate, in-time adherence rate, and treatment outcome Real-time visualization Unavailable Unavailable Real-time analysis and visualized for adherence of treatment and the follow-up examination results Real-time alert Hard to track patients Hard to track patients Surveillance and alert to the poor compliance patients Abbreviation: TBIMS=Tuberculosis Information Management System; CMS=case management system; MDR-TB=multidrug-resistant tuberculosis; SQL=structured query language. Table 1. The comparison of different features between the paper-based reports, the national TBIMS and the CMS for MDR-TB.
-
The pilot study for the application of the CMS was initiated in the Yunnan Tuberculosis Clinical Center (TCC) in 2017. Then, this study applied a simple randomized sampling method: sampling 5 prefectures (Baoshan, Honghe, Lincang, Dehong and Pu’er), from 16 overall prefectures of Yunnan Province, who implemented the pilot between 2018 and 2019. Another 11 prefectures of Yunnan were defined as non-pilot sites. Meanwhile, 5 cities (Jinan, Urumqi, Zhenjiang, Yichang, Wuhan) in 4 provincial-level administrative divisions (PLADs) carried out the CAP-TB program synchronously.
-
The real-time RFUR was extracted from the CMS; then, real-time RFUR was evaluated in TCC and 5 sites of the pilot study between 2017 and 2020, respectively. The coverage and use of the CMS in Yunnan was calculated between 2017 and 2020.
Data from the pilot and non-pilot sites of the MDR-TB treatment cohort between 2017 and 2019 were extracted from the TBIMS. The LTFU rates for pilot and non-pilot sites were compared by a chi-square test. The potential factors associated with LTFU for MDR-TB treatment cohorts were categorized and described by proportion, then assessed by univariate and multivariate binary logistic regression.
Statistical analyzes were done by using R software (version 4.0.2, R Core Team, Vienna, Austria). The statistical significance level was set as P<0.05.
-
At the pilot sites, the CSC model for MDR-TB was implemented with supportive care services put in place. Based on the CMS data from the Yunnan pilot sites, the average MDR-TB RFUR was 90.7% in TCC and 73.7% at the prefecture-level sites (Figure 2A).
 Figure 2.
Figure 2.The MDR-TB regular follow-up rate and loss to follow-up rate in treatment cohort, and the factors associated with loss to follow-up in Yunnan, 2017–2020. (A) Illustration of the MDR-TB regular follow-up rate in TCC and pilot sites of Yunnan, 2017–2020. (B) Comparison of loss to follow-up rate between the MDR-TB treatment cohorts in the pilot and non-pilot sites of Yunnan, 2017–2019. (C) Crude odds ratio of the factors associated with the loss to follow-up for the MDR-TB patients in Yunnan, 2017–2019. (D) Adjusted odds ratio of the factors associated with the loss to follow-up for the MDR-TB patients in Yunnan, 2017–2019.
Note: The asterisk in Figure 2B presented that the loss to follow-up rate was significantly different in the pilot and non-pilot treatment cohort (P<0.05). The point and interval in Figure 2C–D presented the univariate and multivariate logistic regression odds ratio and its 95% confidence interval, respectively. The solid circle was regression coefficient P<0.05, otherwise, the hollow circle was regression coefficient P≥0.05.
Abbreviation: CMS=case management system; MDR-TB=multidrug-resistant tuberculosis; TCC=tuberculosis clinical center; mWRDs=molecular WHO-recommended rapid diagnostic tests; cDST=conventional drug susceptibility test.
In 2020, use of the CMS was scaled up to all 16 prefectures of Yunnan Province. With the YNCDC endorsement, utilization of the CMS has become the standard of care for MDR-TB control in the province. The coverage rate of the CMS reached 100% in Yunnan. The CMS was also extended beyond Yunnan to reach four additional PLADs in China.
Under the CSC model, the patient-centered CMS contributed to improved patient regular follow-up of the MDR-TB treatment. The average LTFU rate was 9.0% for the treatment cohort in pilot sites, which was significantly lower (P<0.05) than non-pilot sites (20.6%) between 2017 and 2019 (Figure 2B).
Across the 816 patients in the MDR-TB treatment cohort (Table 2), the risk of LTFU reduced by 61.7% during CMS pilot implementation [adjusted odds ratio (aOR): 0.38, 95% confidence interval (CI): 0.23–0.60] compared with non-pilot sites (Figure 2C–D).
Characteristic Loss to follow-up Other treatment outcome Overall χ2 P N (%) N (%) N (%) Overall 132 (16.2%) 684 (83.8%) 816 (100.0%) Treatment cohort 2017 20 (15.2%) 124 (18.1%) 144 (17.6%) 0.89 0.64 2018 45 (34.1%) 238 (34.8%) 283 (34.7%) 2019 67 (50.8%) 322 (47.1%) 389 (47.7%) Intervention Non-pilot 104 (78.8%) 401 (58.6%) 505 (61.9%) 19.07 <0.01 CMS pilot 28 (21.2%) 283 (41.4%) 311 (38.1%) Sex Male 91 (68.9%) 480 (70.2%) 571 (70.0%) 0.08 0.77 Female 41 (31.1%) 204 (29.8%) 245 (30.0%) Age (years) <35 39 (29.5%) 233 (34.1%) 272 (33.3%) 1.02 0.60 35–64 83 (62.9%) 403 (58.9%) 486 (59.6%) ≥65 10 (7.6%) 48 (7.0%) 58 (7.1%) Ethnicity Han 102 (77.3%) 462 (67.5%) 564 (69.1%) 4.91 0.03 Other minorities 30 (22.7%) 222 (32.5%) 252 (30.9%) Occupation Farmer 104 (78.8%) 532 (77.8%) 636 (77.9%) 0.07 0.79 Others 28 (21.2%) 152 (22.2%) 180 (22.1%) Type of case New 44 (33.3%) 237 (34.6%) 281 (34.4%) 0.08 0.77 Retreatment 88 (66.7%) 447 (65.4%) 535 (65.6%) DST mWRDs 100 (75.8%) 498 (72.8%) 598 (73.3%) 0.49 0.48 cDST 32 (24.2%) 186 (27.2%) 218 (26.7%) Abbreviation: MDR-TB=multidrug-resistant tuberculosis; CMS=case management system; DST=drug susceptibility test; mWRDs=molecular WHO-recommended rapid diagnostic tests; cDST=conventional drug susceptibility test Table 2. The characteristics of the MDR-TB treatment cohort categorized by treatment outcome of Yunnan, 2017–2019.
-
In the fight against the MDR-TB epidemic, it is critical to strengthen effective MDR-TB detection and ensure treatment completion as the world works towards reaching a cure. Poor treatment adherence and undesirable outcomes exacerbate transmission of MDR-TB across populations. The CSC model and CMS filled the gap by providing patient-centered, individualized care to improve treatment adherence. The CMS responded to the needs of the patients and managed to tackle critical challenges in MDR-TB control.
Embedded in the three-in-one system of TB care delivery, the CMS strengthened communication and collaboration between CDCs, designated hospitals, and community healthcare service institutions. As a platform that encourages real-time cooperation and information sharing, CMS strengthened coordination between different TB stakeholders.
The CMS integrated and unified fragmented clinical data spread across different systems, enabling users to access all key strategic information in one interface. The CMS also provided a multi-dimensional view of the patient's entire medical history. With the CMS in place, case management of MDR-TB was optimized with increased precision, timeliness, and effectiveness. Use of the CMS has been demonstrated to be an effective measure for data-driven quality assurance and quality improvement of the supportive care delivered to the patients. The powerful real-time analysis function, visualization, and tracking of key indicators allowed users to promptly identify and address potential barriers to treatment adherence.
Previous studies provided evidence that digital technology could promote treatment adherence and improve outcomes for TB. A systematic review showed that treatment outcomes among drug-susceptible tuberculosis can be strengthened through effective adherence interventions such as patient education and counseling; management of incentives and enablers; provision of psychological interventions, reminders and tracers; and use of digital health technologies (5). Another systematic review showed that medication monitoring increased the probability of cure (relative risk 2.3, 95% CI: 1.6–3.4) (6). Although short message service (SMS) and video-observed therapy (VOT) as interventions reported comparable treatment completion when compared with directly observed treatment (DOT), more evidence and high-quality studies are needed to illustrate how such digital technology could strengthen patient compliance (7–8).
This study was subject to some limitations. Although the data of the Yunnan cohort was used for the evaluation of the CMS, more evidence is needed for the generalization of the CMS across different settings. Application scenarios for the CMS should still be according to specific health care resource allocation, the diverse barriers of MDR-TB treatment, and CSC model best practices. The CMS is just a tool and a platform for strengthening MDR-TB case management to meet the requirements of the CSC model and supportive care for MDR-TB treatment. There also remains a need for more investigations to understand the association between adherence intervention and favorable outcomes of MDR-TB. Next, the exchange of data between the CMS and other systems sometimes proves difficult. The CMS thus needs to be further technically enhanced to increase its compatibility and connectivity with existing systems, such as the National Notifiable Disease Reporting System (NNDRS), the TBIMS, hospital information systems (HIS), laboratory information systems (LIS), and the National Project of Basic Public Health Service (BPHS). A comprehensive information system could close such gaps in digital technology. This more advanced system should be underpinned by the concepts of case management and patient-centered care, and should be able to monitor and manage lifelong disease and health issues through a unified dataset that can automatically exchange and share information (9).
-
TB professionals in Yunnan Center for Disease Control and Prevention, FHI 360, related health care providers in other pilot PLADs (Shandong, Xinjiang, Jiangsu, Hubei), and Anh L. Innes.
HTML
Development of the CMS
Features of the CMS
The Pilot Study of the CMS
Data Analysis
| Citation: |



 Download:
Download:






