-
Coronavirus disease 2019 (COVID-19), caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), has become a severe threat to global public health (1). The virus has spread rapidly to more than 200 countries worldwide. The World Health Organization (WHO) declared the outbreak of COVID-19 to be a global pandemic on March 11, 2020. As of February 15, 2022, the disease had caused more than 400,000,000 human infections with over 5,000,000 deaths globally (2). Moreover, the constant emergence of variants of concern (VOCs) of SARS-CoV-2, such as the Omicron (B.1.1.529) variant, increases the risk of vaccine failure (3). Thus, there is an urgent need for the development of antiviral drugs.
Type I interferons (IFNs) α/β are one of the most common biotechnological drugs that have broad-spectrum antiviral activities against ribonucleic acid (RNA) viruses. Type I IFNs induce an antiviral response across a wide range of cell types and mediate adaptive immune responses (4). SARS-CoV-2 replication is inhibited by IFN-α and IFN-β in vitro (5). Novaferon (Nova) is a new recombinant IFN-α-like protein with significantly higher activity than IFN-α; it has been approved for the treatment of chronic hepatitis B in China (6). In our previous study, Nova was shown to inhibit ancestral SARS-CoV-2 replication in vitro (7). The Nova and Nova plus lopinavir/ritonavir groups had significantly higher viral clearance rates than the lopinavir/ritonavir group and a 3-day reduction in viral clearance (7). The cytotoxic effect of Nova was assayed in this study, and we reported that Nova exhibited antiviral activity not only against ancestral SARS-CoV-2, but also against the Omicron variant in cultured cells. These results showed the therapeutic potency of type I IFNs against COVID-19.
-
African green monkey kidney Vero cells (ATCC, CCL-81) were cultured at 37 °C in Dulbecco’s modified Eagle’s medium (DMEM) (Gibco, Grand Island, NY, USA) supplemented with 10% fetal bovine serum(FBS)(Gibco, Grand Island, NY, USA), 200 mg/mL streptomycin, and 200 IU/mL penicillin in an atmosphere containing 5% CO2. SARS-CoV-2 viruses (ancestral virus 2019nCoV-CDC-Tan-HB01 and the Omicron variant 2019nCoV-CDC-Tan-GD01) were kept in our laboratory. The viruses were propagated in Vero cells. Viral titers were determined using a standard TCID50 assay. All infection experiments were performed in a biosafety level-3 laboratory.
The cytotoxicity of Nova was determined in Vero cells using CCK8 assays (DOJINDO, Kumamoto, Japan). Briefly, Vero cells were seeded in 96-well plates and cultured overnight. Different concentrations of the compound solution (100 μL) in DMEM were added to the Vero cells and incubated for 48 h at 37 °C with 5% CO2; 10 microliters of reagent from CCK8 assays were added to each well 48 h after incubation. The OD450 value was measured using a microplate reader (TECAN Infinite 200 Pro, Switzerland) after 1 h of incubation at 37 °C. The experiments were performed in triplicate. The OD450 value in the presence of different concentrations of the compound was divided by the OD450 value of the negative control to calculate the percentage of cytotoxicity. Cytotoxicity curves were plotted using GraphPad Prism 5.0 (GraphPad Software Inc., San Diego, CA, USA).
The antiviral activities of Nova and remdesivir against SARS-CoV-2 were evaluated in vitro. Briefly, cells were seeded in 96-well plates at a density of 2×104 cells/well and grown for 24 h. Vero cells were infected at a multiplicity of infection (MOI) of 0.01 for 1 h at 37 °C. Virus was washed with DMEM twice and then cells were treated with a medium containing Nova at various concentrations or remdesivir at different concentrations (20 μmol/L, 4 μmol/L, 0.8 μmol/L, 0.16 μmol/L, 0.032 μmol/L) for 48 h. For the prophylactic administration test, Nova was added 2 h before viral infection and washed twice with DMEM. The cells were then incubated in fresh DMEM for 48 h. The supernatant was collected, and the RNA was extracted and analyzed by relative quantification by quantitative real-time reverse transcription polymerase chain reaction (qRT-PCR), as described in a previous study (8).
Viral RNA was extracted from 100 μL supernatant of infected cells using an automated nucleic acid extraction system (TIANLONG, Xi'an, China) following the manufacturer’s recommendations. SARS-CoV-2 was detected using the One Step PrimeScript RT-PCR kit (TaKaRa, Shiga, Japan) on a LightCycler 480 Real-Time PCR system (Roche, Rotkreuz, Switzerland). ORF 1ab was amplified from cDNA, cloned into MS2-nCoV-ORF1ab, and used as the plasmid standard after its identity was confirmed by sequencing. A standard curve was generated by determining copy numbers from serial dilutions (103–109 copies) of the plasmid. The primers used for quantitative PCR were 1ab-F: 5’-AGAAGATTGGTTAGATGATGATAGT-3’; 1ab-R: 5’-TTCCATCTCTAATTGAGGTTGAACC-3’, and probe 5’-FAM-TCCTCACTGCCGTCTTGTTGACCA-BHQ1-3’. The individual concentration for 50% of maximal effect (EC50) values was calculated using GraphPad Prism 5.0. All experiments were conducted in triplicate.
-
Cell viability after Nova treatment was determined using the CCK8 assay in Vero cells. The 50% cytotoxic concentration (CC50) of Nova was 1,076 ng/mL (Figure 1A). To investigate the antiviral effect of Nova against the ancestral SARS-CoV-2 virus, Vero cells were infected with the virus and incubated with Nova and remdesivir at various concentrations for 48 h. Remdesivir was selected as the positive control in our study, and the results showed that the EC50 of remdesivir was 1.52 µmol/L (Figure 1B). Nova inhibited the replication of the SARS-CoV-2 ancestral virus with an EC50 value of 0.0019 ng/mL (Figure 1C).
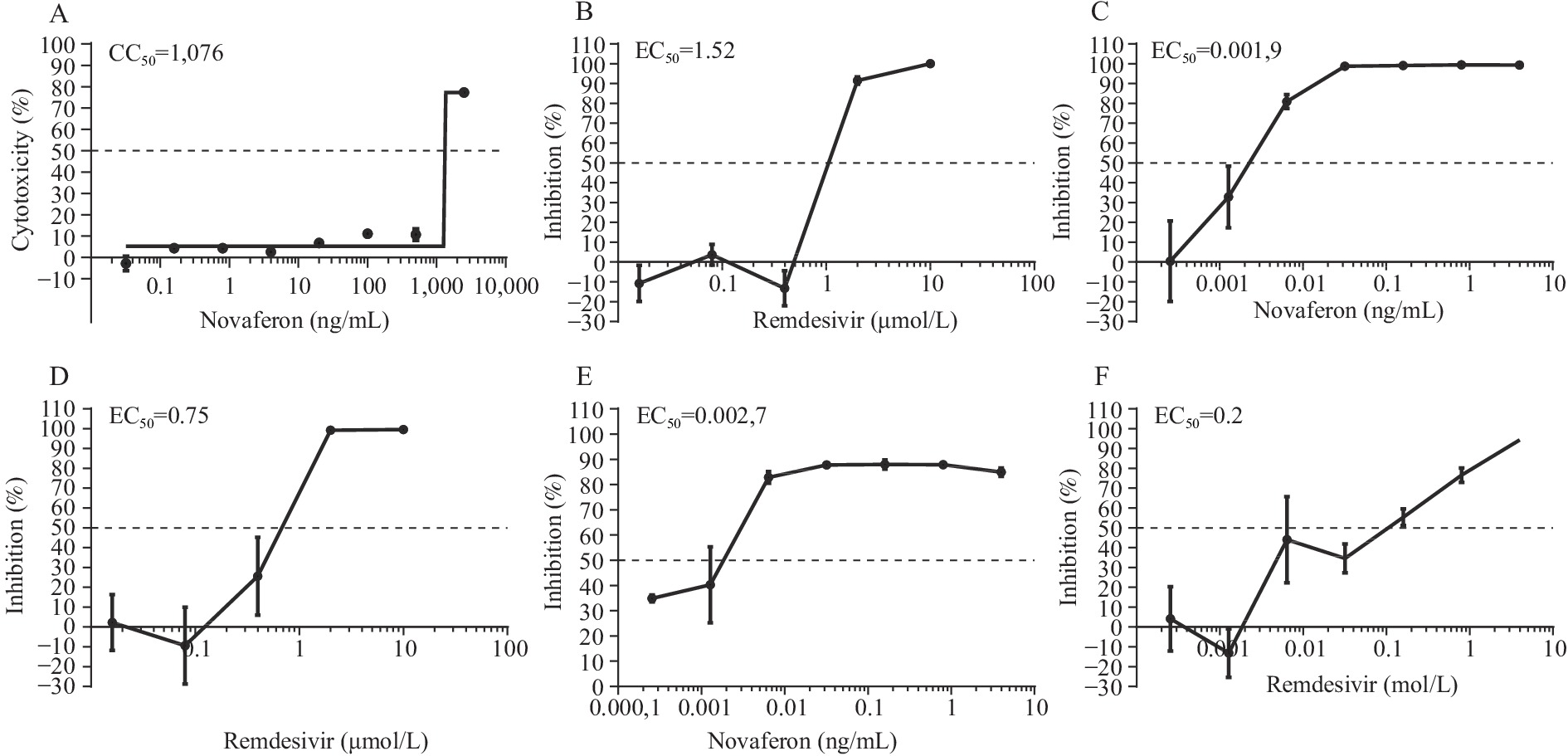 Figure 1.
Figure 1.Cytotoxic effect of Nova and antiviral activities of Nova and remdesivir against ancestral SARS-CoV-2 and the Omicron variant in Vero cells.
(A) The cytotoxicity of Nova. (B and C) Antiviral activities of remdesivir and Nova against ancestral SARS-CoV-2. (D and E) Antiviral activities of remdesivir and Nova against the Omicron variant. (F) Prophylactic antiviral activity of Nova against the Omicron variant.
Abbreviations: Nova=Novaferon; SARS-CoV-2=severe acute respiratory syndrome coronavirus 2.
To illustrate the efficacy of Nova in inhibiting the SARS-CoV-2 Omicron variant replication in vitro, Vero cells were infected with the Omicron variant and incubated with Nova and remdesivir at various concentrations for 48 h. Results showed that remdesivir inhibited the Omicron variant with an EC50 of 0.75 µmol/L (Figure 1D). The EC50 values of Nova for SARS-CoV-2 Omicron variant were 0.0027 ng/mL (Figure 1E).
In Vero cells, pretreatment with different concentrations of Nova influences the replication of the SARS-CoV-2 Omicron variant. The EC50 of pretreatment with Nova for the SARS-CoV-2 Omicron variant was 0.2 ng/mL (Figure 1F). Taken together, these results indicated that pretreatment or treatment with type I IFN significantly inhibited both ancestral SARS-CoV-2 and Omicron variant infections in vitro.
-
Owing to the lack of specific antiviral drugs, rapid evaluation of the antiviral activity of existing licensed drugs is a critical method to combat the pandemic. Here, we showed that Nova inhibits replication of ancestral SARS-CoV-2 and the Omicron variant in vitro. Pretreatment with Nova protected cells against Omicron variant infection in vitro.
Type I IFNs are the first line of defense and are vital for blocking early viral replications, spread, and tropism, as well as promoting the adaptive immune response. Type I IFNs induce a systemic response that affects almost every cell in the host (4). It is reported that compared to the severe acute respiratory syndrome coronavirus, SARS-CoV-2 is more sensitive to IFN-I (9). In addition, IFN beta-1b was shown to decrease virus-induced lung fibrosis in a mouse model (10), which may improve the outcomes of patients with COVID-19 complicated by acute respiratory distress syndrome. These results were partly consistent with those of our previous study: Nova and Nova plus lopinavir/ritonavir groups had significantly higher viral clearance rates than the lopinavir/ritonavir alone or control groups (7).
As the SARS-CoV-2 Omicron variant replaced the Delta variant as the main pandemic virus in late 2021 in the world, the risk of severe breakthrough infection was high (3). Considering immune-compromised individuals and the high price of monoclonal antibody therapy and antiviral agents, more treatment options were urgently needed. The IFNs were proved to be safe and available, more clinical trials on IFNs alone or combined with other medicines were worth exploring.
However, there were limitations in this study. The inhibition of Nova against the ancestral SARS-CoV-2 and Omicron variant was only tested in vitro. The in vivo effects of Nova need to be tested in animal models such as K18-hACE2 mice. And more importantly, large-scale double-blinded clinical trials are needed to verify the efficacy of Nova in COVID-19.
In summary, we characterized the inhibition of Nova against ancestral SARS-CoV-2 infection in vitro. Antiviral activity of Nova was also observed in SARS-CoV-2 Omicron variant-infected cells. Furthermore, pretreatment of Vero cultures with Nova reduced SARS-CoV-2 replication. Overall, these data suggested that Nova is a potential candidate for the management of COVID-19 and could be worthy of further in vivo study and clinical trial.
-
No conflicts of interest.
HTML
| Citation: |




 Download:
Download:




