-
From 1950 to 2019, a total of 267 plague cases in humans were reported in the Inner Mongolia Autonomous Region with 133 deaths and 10,710 Yersinia pestis isolates; 21 of these cases were reported from the Meriones unguiculatus (M. unguiculatus) plague focus with 6 deaths and 6,771 isolates. According to the plague situation in the Inner Mongolia Autonomous Region and the implementation of local preventive measures, the prevalence of plague in humans in the M. unguiculatus plague focus of the Inner Mongolia Autonomous Region could be divided into four stages. The first stage was the initial stage of plague prevention and control (1950–1959), with 0.80 cases annually and a case fatality rate of 37.50%. The second stage was the plague eradication stage (1960–1979), with 0.15 cases annually and a case fatality rate of 33.33%. The third stage was the plague surveillance stage (1980–1999), with 0.25 cases annually and a case fatality rate of 20.00%. The fourth stage is the comprehensive prevention and control stage under the emergency system (2000–2019), with 0.25 cases annually and a case fatality rate of 20.00%. The surveillance of rodent density (1981–2019) and studies on plague-related factors among M. unguiculatus have shown that the higher the M. unguiculatus density, the higher the nocturnal rodent capture rate (r=0.670, p<0.05) and the higher the indirect hemagglutination assay (IHA) positive rate of M. unguiculatus (r=0.344, p<0.05); the higher the percentage of hosts infected, the lower the M. unguiculatus density (r=–0.361, p<0.05) and the lower the IHA positive rate of M. unguiculatus (r=–0.337, p<0.05); the higher the percentage of nests infected with fleas, the lower the IHA positive rate of M. unguiculatus (r=–0.348, p<0.05). Together, these results suggest that it is necessary to simultaneously monitor the pathogens, serology, and vector index of M. unguiculatus to accurately reflect the plague prevalence among local animals. Although bubonic plague is the main plague type of the M. unguiculatus plague focus, severe pneumonic plague or septic plague may be secondary when the bubonic plague is misdiagnosed or not treated in time. In addition, the plague in animals is relatively virulent in the M. unguiculatus plague focus, and the risk of spreading to humans is higher. For plague in the Inner Mongolia Autonomous Region, comprehensive control efforts should be aimed at the M. unguiculatus focus, covering host animals, vector insects, and humans.
-
Plague, which is caused by Yersinia pestis (Y. pestis), is one of the most severe infectious diseases that endangers humans (1). There have been three pandemics of the plague in humans causing hundreds of millions of deaths and indirectly affecting the development of human society and resulting in dynastical changes (2). Currently, Madagascar, the Democratic Republic of the Congo, and Peru are the three countries most affected by plague (3-5).
The Inner Mongolia Autonomous Region is located in northern China and borders Heilongjiang, Jilin, Liaoning, and Hebei to the northeast; Shanxi, Shaanxi, and Ningxia to the south; Gansu to the southwest; and Russia and Mongolia to the north (6). From 1901 to 1949, 41 epidemics of plague in humans and 80,000 deaths were recorded within 470,000 square kilometers of the Inner Mongolia Autonomous Region. Since the 1960s, no large epidemic has occurred, and only sporadic cases have occurred. However, because of the potential transmission risk, plague in the Inner Mongolia Autonomous Region is still of great concern (7). According to the characteristics of the geographic landscape, host, vector, and Y. pestis ecotype, 12 types of plague foci were identified in China (8). There are four types of plague foci in the Inner Mongolia Autonomous Region: the Marmota sibirica (M. sibirica) plague focus on the Hulun Buir Plateau, the Spermophilus dauricus (S. dauricus) plague focus on the Song-Liao Plain, the Misrotus brandti (M. brandti) plague focus on the Xilin Gol League Plateau, and the Meriones unguiculatus (M. unguiculatus) plague focus on the Inner Mongolian Plateau. For the M. sibirica plague focus, no cases have occurred since 1923, when Wu Liande detected Y. pestis from M. sibirica near Manzhouli. Afterwards, M. sibirica became nearly extinct in China due to mass hunting for its fur, and no animal or plague in humans occurred after 1926 in China (9-12). In recent years, because of hunting bans and farmland being reclaimed to grassland, M. sibirica has gradually migrated from Mongolia to China, and its density has increased. Since 2019, Mongolia has reported more than 20 suspected human cases in the M. sibirica plague focus. Thus, the plague in animals and humans of Mongolia may have spread to China (13).
Before 1960, cases in humans in the Inner Mongolia Autonomous Region were mainly distributed in the S. dauricus plague focus of the Song-Liao Plain. After the 1960s, no human cases have been reported in this focus because large-scale rodent eradication has controlled plague in animal. For the M. brandti plague focus of the Xilin Gol League Plateau, the most prominent feature is that the Y. pestis strain is not pathogenic to humans and is weakly virulent. Presently, the major risk of plague in humans in the Inner Mongolia Autonomous Region is within the M. unguiculatus plague focus of the Inner Mongolian Plateau. At the beginning of the 20th century, human bubonic plague occurred multiple times within the activity area of M. unguiculatus. The M. unguiculatus plague focus was first identified in 1954 in Hangjinhouqi and Linhe County, where Y. pestis was isolated from dead M. unguiculatus and their fleas in burrows (14-15). There were active of plague in animals almost every year. The primary vector species was Nosopsyllus laeviceps kuzenkovi which had the ability to bite both humans and rodents(7). Although the main type of plague caused by M. unguiculatus in humans is bubonic plague, in 2019, two cases of the M. unguiculatus plague focus from the Xilin Gol League in the Inner Mongolia Autonomous Region were diagnosed as pneumonic plague in Beijing Chaoyang Hospital (16). Although the cases did not cause a large-scale spread in 2019, the highly contagious characteristics of pneumonic plague (17) suggested potential risks of large-scale outbreaks of the M. unguiculatus plague focus of the Inner Mongolian plateau, which is the key region for plague prevention and control. Herein, we analyzed the characteristics and distribution of plague in humans and plague in animals from 1950 to 2019.
-
The data, collected from the Inner Mongolia Autonomous Region, included the year of the distribution of plague in humans and the number of cases and mortality from 1950 to 2019; the status of plague in animals, mainly including the host distribution profile and the number of isolated strains from 1950 to 2019; and the influencing factors for the plague in animals, etiology and serology test results, and the vector index from 1981 to 2019.
Plague cases in humans before 1958 were confirmed by clinical manifestations and epidemiological investigations, such as sudden high fever and lymphadenopathy, usually accompanied by rodent deaths in the early stages of the disease. Since 1958, the confirmation of Y. pestis isolations and plague cases has been based on microbiological and serological diagnoses, especially in the diagnosis of plague cases in humans WS 279-2008 “Diagnostic Standards of Plague” after 2008 (18).
The method of fixed surveillance sites and mobile surveillance sites was adopted to monitor plague among animals. For fixed surveillance sites, 300 to 500 animals were assessed by bacteriological methods at each site; for mobile surveillance sites, not less than 100 animals were assessed at each site; all fleas (ticks) collected were classified. Except for a few fleas and ticks that were used as specimens, the rest of the collected fleas and ticks were classified and evaluated for pathogens. Flea species and location were recorded based on the host, and each group of 10 to 20 fleas or ticks was allocated for isolation, culture, and testing against Y. pestis. When an epidemiological indication of plague was found, the search scope was expanded and the number of inspections was increased. Area surveillance focused on a large-scale search of dead rodents for pathogenic testing.
Culture and staining of isolates were performed as follows: tested isolates were cultured on Hexcel’s ordinary agar, Hexagon’s blood agar, broth, and 3% NaCl slant and stained with gram staining to observe the typical morphology of Y. pestis.
Bacterial determination was completed according to the relevant methods in the “Handbook of Plague Prevention and Control” and the conventional “four-step inspection method” of plague bacteriology (microscopic examination, isolation culture, plague phage lysis test and animal test) to determine the strain (19).
Serological testing of F1 antibodies was assayed with indirect hemagglutination. At a fixed surveillance site, no fewer than 100 sera from rats, the main hosts that are also highly resistant rodents, were tested as the main surveillance objects. Mobile surveillance sites were used to detect live rats that were captured. Antibody detection was used for epidemiological investigation, surveillance, diagnosis, and tracing of plague in humans as well as assessment of the level of plague infection in live rats.
The density of M. unguiculatus was calculated with the following formula: number of M. unguiculatus caught/square quadrat (hm2). The number of M. unguiculatus was counted using the 24-hour bow clamp method.
The calculation method for monitoring fleas was as follows: host-flea index = total number of fleas from the rats / number of rats inspected. The percentage of hosts infested with fleas (%) = number of rats with fleas / total number of rats inspected × 100%. The index of nomadic fleas at the passage of burrows = total number of nomadic fleas at the passage of burrows / number of rat burrows inspected. The percentage of the passage of burrows infested with nomadic fleas (%) = number of flea-infested passages of burrows / number of burrows inspected × 100%. The nest-flea index = total number of nest fleas collected / number of nests inspected. The percentage of nests infested with fleas (%) = the number of nests infested with fleas / the number of nests inspected × 100% (19).
Descriptive epidemiology was used to analyze the research data. Excel 2007 and SPSS (23.0, IBM Corp., New York, US) were used to process and analyze the data, and the chi-squared test was used for statistical analysis of categorical variables. Correlation analysis was performed using Pearson Product-Moment Correlation. In the generalized linear model (GLM), the IHA positive rate of M. unguiculatus was used as the dependent variable, while the surveillance items of plague among animals were included as covariates. Fitting of GLM was mainly based on maximum likelihood principles, and the Wald-type test for comparing standardized estimates to the standard normal distribution was used. The difference was considered to be statistically significant at p<0.05.
-
In the Inner Mongolia Autonomous Region, 267 plague cases in humans and 133 deaths were reported from 1950 to 2019, of which 21 plague cases in humans and 6 deaths were reported in the M. unguiculatus plague focus of the Inner Mongolian Plateau. From 1950 to 1959, 254 plague cases in humans were reported. Among them, only 8 cases in humans were reported in the M. unguiculatus plague focus of the Inner Mongolian Plateau, with an average annual incidence of 13 cases in humans and a fatality rate of 51.18%. From 1960 to 2019, 13 human cases were reported, all of which came from the M. unguiculatus plague focus of the Inner Mongolian Plateau with an average annual incidence of 0.22 plague cases in humans and a fatality rate of 23.08% (Figure 1A).
According to the status of plague in the Inner Mongolia Autonomous Region and the implementation of local preventive measures, the epidemic in the M. unguiculatus plague focus of the Inner Mongolian Plateau could be divided into four stages (20–21). The first stage was from 1950 to 1959 and was called the initial stage of plague prevention and control. In this stage, there were 8 cases in humans in the M. unguiculatus plague focus of the Inner Mongolian Plateau. The average annual incidence was approximately 0.80 cases in humans, and the fatality rate was 37.50%; human cases were limited to Hangjinhou. The second stage was from 1960 to 1979 and was called the plague eradication stage. In this stage, the average annual incidence was approximately 0.15 cases in humans, and the fatality rate was 33.33%. The cases were distributed in Sunitezuo, Wulatezhong, and Shangdu. The third stage was from 1980 to 1999 and was called the plague surveillance stage. In this stage, the average annual incidence was approximately 0.25 plague cases in humans, and the average mortality rate was 20.00%. The cases were distributed in Wulateqian, Etuokeqian, and Siziwang. The fourth stage was from 2000 to 2019 and was called the comprehensive plague prevention and control stage under the emergency system. The average annual incidence was approximately 0.25 plague cases in humans, and the average mortality rate was 20.00%. The cases were distributed in four adjacent counties, namely, Suniteyou, Sunitezuo, Siziwang, and Xianghuang (Figure 1A, Figure 2 and Table 1).
-
From 1950 to 2019, a total of 10,710 isolates of Y. pestis were detected in the Inner Mongolia Autonomous Region, with an average of approximately 157.5 isolates every year. The year with the most isolates was 1970, when 1,564 isolates of Y. pestis were discovered. From 1950 to 2019, a total of 6,771 isolates from the M. unguiculatus plague focus of the Inner Mongolian Plateau were detected, with an average of approximately 99.57 isolates each year. The year with the most isolates was 1970, when 1,131 isolates of Y. pestis were discovered (Figure 1B).
From 1981 to 2019, a total of 47,321.95/hm2 were investigated for density surveillance of M. unguiculatus, and 186,073 M. unguiculatus were captured. The average M. unguiculatus density was 3.93/hm2. The densities of M. unguiculatus were the highest in 1984 and 1985 (10.11/hm2 and 12.64/hm2, respectively). A total of 998,162 nocturnal rodents were investigated, and the number of nocturnal rodents captured was 39,711. The nocturnal rodent capture rate was 3.98%. The average percentage of hosts infested with fleas was 25.56%, and the average host-flea index was 0.67. The average percentage of nests infested with fleas was 57.91%, and the average nest-flea index was 8.31. A total of 301,960 rats were autopsied for pathogens in the M. unguiculatus plague focus of the Inner Mongolian Plateau, among which 2,632 were positive, with an average positive rate of 0.87%. A total of 100,920 rats were tested for serum F1 antibodies, among which 1,068 were positive; thus, the average IHA positive rate of M. unguiculatus was 1.06% (Figure 1C, Table 2).
-
In this study, the pathogenic test results, serological test results, and vector index of the M. unguiculatus plague focus of the Inner Mongolian Plateau from 1981 to 2019 were analyzed for correlations between pairs of variables. The results showed a significant positive correlation between the positive rate of M. unguiculatus and the flea group (r=0.596, p<0.05); that is, within a certain range, the positive rate of the flea group increased, and the positive rate of M. unguiculatus also increased. The results also showed the following correlations: a significant positive correlation between the density of M. unguiculatus and the nocturnal rodent capture rate (r=0.670, p<0.05); a significant negative correlation between the density of M. unguiculatus and the percentage of hosts infested with fleas (r=−0.361, p<0.05); a significant positive correlation between the IHA positive rate of M. unguiculatus and the nocturnal rodent capture rate (r=0.344, p<0.05); a significant negative correlation between the IHA positive rate of M. unguiculatus and the percentage of hosts infested with fleas (r=−0.337, p<0.05); and a significant negative correlation between the IHA positive rate of M. unguiculatus and the percentage of nests infested with fleas (r=−0.348, p<0.05) (Figure 3).
In this study, the IHA positive rate of M. unguiculatus in the M. unguiculatus plague focus of the Inner Mongolian Plateau from 1981 to 2019 was used as the dependent variable. Density of M. unguiculatus (/ha), nocturnal rodent capture rate, percentage of hosts infested with fleas (%), host-flea index, percentage of the passage of burrows runways infested nomadic fleas (%), the index of nomadic fleas at the passage of burrows, percentage of nests infested with fleas (%), and nest-flea index were included as covariates in the GLM. The results showed that there were statistical correlations between the IHA positive rate of M. unguiculatus and the host-flea index (χ2=5.394, p<0.05), the percentage of nests infested with fleas (χ2=46.991, p<0.05), the nest-flea index (χ2=46.320, p<0.05), the percentage of the passage of burrows infested with nomadic fleas(χ2=11.680, p<0.05), and the index of nomadic fleas at the passage of burrows (χ2=4.576, p<0.05) (Table 3).
-
The M. unguiculatus plague focus of the Inner Mongolian Plateau was first confirmed in 1954 (14–15) and is located on the Ulanqab Plateau, in desert grassland on the Ordos Plateau, and in grassland on the Chahar Hills, which are all located at an altitude of more than 1,000 meters. The plague focus is distributed in more than 30 counties in Inner Mongolia, Ningxia, Shaanxi, and Hebei provinces(6). The M. unguiculatus are a species of Mongolian gerbils that are widely distributed in Inner Mongolia and are the representative dominant species in the desert steppe. In nature, they are distributed in one of three patterns: island, banded, and dispersed distributions. In its densest distribution areas, M. unguiculatus can account for up to 79.8% of all rodents species (7). Mongolian gerbils exhibit no hibernation period and are active all year round, resulting in a lack of significant seasonality in plague cases in humans. However, because the life span of M. unguiculatus is only 1–1.5 years, the effect of interanimal immunity caused by plague epidemics among animals is not clear, which is the main characteristic of M. unguiculatus-dependent plague. The main vector insects in this focus are Nosopsyllus laeviceps kuzenkovi, Xenopsylla conformis conformis, and Neopsylla pleskei orientalis (7). The peak season for fleas in M. unguiculatus is the warm season, and the peak season for fleas in the nest is the cold season; thus, fleas infected with plague can exist all year round, especially in winter. The infected fleas have a strong affinity for human blood and can carry Y. pestis throughout the winter. Unlike marmots, which live for more than 10 years, M. unguiculatus have a shorter lifespan, meaning that frequent host renewal occurs during epidemics, which leads to a much weaker immune barrier established during epidemics than in other rodents. The maximum titer of serum antibody in M. unguiculatus was 1∶2,560 under laboratory conditions. However, the serum positive rate was 10%, and the antibody titer was 1∶10,240 for M. unguiculatus caught in an area suffering from recurrent plague epidemics (7). It is precisely because of these characteristics in this focus that the epidemic regularity and epidemic intensity of the M. unguiculatus plague are difficult to predict, making the formulation and implementation of prevention and control measures somewhat difficult.
There was a positive significant correlation between the density of M. unguiculatus and the nocturnal rodent capture rate (r=0.670, p<0.05); a significant correlation between the IHA positive rate of M. unguiculatus and the nocturnal rodent capture rate (r=0.344, p<0.05). Thus, it is suspected that there was an indirect correlation between the density of M. unguiculatus and the IHA positive rate of M. unguiculatus. It is inferred that the density of M. unguiculatus was higher, and the IHA positive rate of M. unguiculatus was higher within a certain range. However, there was a significant negative correlation between the density of M. unguiculatus and the percentage of hosts infested with fleas (r=–0.361, p<0.05), which suggests that an increase in the percentage of hosts infested with fleas would increase the exposure of M. unguiculatus to infected fleas, thereby increasing the probability of plague infection of M. unguiculatus and decreasing the density of M. unguiculatus. This conclusion is consistent with the surveillance data in Southwest China from 2000 to 2015, in which the rat density decreased and the flea rate increased simultaneously (22). Moreover, the density of M. unguiculatus increased in 1984 and 1985 and then dropped in 1986 and 1987.
Surprisingly, plague in humans also occurred in 1986 and 1987 (Figure 1A and 1C). It can be hypothesized that as the density of rats increases, the contact between rats will increase, and the plague will spread among animals. The increase in dead rats caused by the plague reduced the density of rats, which may provide a prerequisite for the occurrence of plague in humans. This thinking is consistent with another study that used rat density as an important parameter in plague prediction models (23).
There was a significant negative correlation between the IHA positive rate of M. unguiculatus and the percentage of hosts infested with fleas (r=−0.337, p<0.05), and a significant negative correlation between the IHA positive rate of M. unguiculatus and the percentage of nests infested with fleas (r=−0.348, p<0.05). These seemingly paradoxical results may stem from the following reasons. The IHA positive rate came from live M. unguiculatus, while the percentage of hosts infested with fleas and the percentage of nests infested with fleas increased, causing the risk of plague infection of M. unguiculatus to increase and the death of infected M. unguiculatus to increase. However, those who survived were often healthy, so the percentage of hosts infested with fleas and the percentage of nests infested with fleas increased, and the IHA positive rate of M. unguiculatus decreased (Figure 3). Therefore, it is necessary to simultaneously conduct pathogenic surveillance, serological surveillance, and vector index analysis of gerbils to accurately reflect the prevalence of plague among local animals.
From 1950 to 1959, the number of cases of plague in Inner Mongolia was relatively high, while the number of cases in the M. unguiculatus plague focus of the Inner Mongolian Plateau was not. The cases of plague appeared and disappeared alternately after 1960 in the M. unguiculatus plague focus of the Inner Mongolian Plateau (Figure 1 and Figure 2). A large number of studies have confirmed that plague foci have alternating periods of rest and activity (22–24). In Inner Mongolia, the M. unguiculatus plague focus of the Inner Mongolian Plateau has alternating periods of rest and activity, while other plague foci of Inner Mongolia are in a resting state. Therefore, this suggests that the target of plague prevention strategies should be the M. unguiculatus plague focus of the Inner Mongolian Plateau. Combined with the data of Y. Pestis isolates, it can be concluded that a large number of isolates can still be detected in the environment and in hosts when the plague focus is at rest, indicating that the plague is still epidemic among animals. In the initial stage of plague prevention and control (1950–1959), the prevention and control of plague and the economy in China were both weak points, and the plague caused by M. unguiculatus was generally mild in human patients, so attention was focused on severe patients. After the 1950s in Inner Mongolia, there were only sporadic cases of the plague in the M. unguiculatus plague focus of the Inner Mongolian Plateau, and the expansion of the plague focus due to land desertification may also have been the reason for the sporadic and increasing cases in this focus. In the plague eradication stage (1960–1979), it was believed that unilateral eradication of the host would eradicate the plague, so intensive rodent extermination efforts were carried out in the plague focus. Although such measures cannot eradicate the plague, the reduction of the host could reduce the risk of the spread of plague to some extent. This may explain the high detection rate of plague in animals in the 1970s but with relatively few human cases. In the plague surveillance stage (1980–1999), China gradually formed a plague surveillance system, and the prevention and control measures were more targeted. At this stage, as during the previous stage, the plague in humans was still sporadic, and the plague in animals continued to be epidemic. In the comprehensive prevention and control stage under the emergency system (2000–2019), the emergency system was added on the basis of surveillance, and more timely and effective measures could be taken to prevent and control the plague. Interestingly, unlike the previous two stages in which the spatial distribution of the plague in humans was relatively dispersed, the cases in the comprehensive prevention and control stage were distributed in four adjacent counties. In 2019, a simultaneous rise in plague cases in humans and plague cases in animals appeared. Therefore, it should be noted that the plague in this focus is excessively active and shows a great risk of transmission.
Plague prevention and control in the Inner Mongolia Autonomous Region of China remain a critical issue. The M. sibirica plague focus of the Hulun Buir plateau, which presents a potential risk of reactivation, has not experienced an epidemic of plague in animals and humans for nearly a hundred years. The S. dauricus plague focus of the Song-Liao Plain, which is in a resting stage, presents a possibility of outbreaks at any time due to an extremely low plague prevalence among animals (22). With the serious desertification of grasslands in recent years, the M. unguiculatus plague focus of the Inner Mongolian Plateau, where enzootic and plague in humans are currently prevalent, has been expanding; and the threat to people has been increasing. Although enzootic plague is continuously prevalent in the M. brandti plague focus of the Xilin Gol League plateau, it has not posed any threat to human beings. With the evolution of the strains, the risk of human infection still exists. Therefore, the comprehensive prevention and control of the Inner Mongolian plague should focus on the M. unguiculatus plague focus of the Inner Mongolian Plateau.
In addition, the Inner Mongolia Autonomous Region is also in danger of importing plague cases from abroad. There are active natural foci in neighboring Mongolia and Russia. The two countries share a border with Inner Mongolia that stretches more than 4,000 kilometers and presents dozens of interchanging ports. In addition, many adjacent areas are natural plague foci that are active or resting for many years. Most of the border lines are not separated by natural barriers. The natural foci are connected as a whole, and enzootic plague can spread among animals (25–29). From 2019 to 2020, more than 20 suspected sporadic cases of plague in humans were reported in natural marmot plague foci in Mongolia (13). This shows that the Mongolian marmot plague is extremely active in Mongolia, and these foci are connected with the M. sibirica plague focus of the Hulun Buir Plateau. Although the M. sibirica plague focus of the Hulun Buir Plateau has been resting for nearly a hundred years, it previously caused disasters in northeast China (9-12). The M. sibirica plague focus of the Hulun Buir Plateau presents potential danger of reactivation. Therefore, there is a double risk of plague resurgence in the M. sibirica plague focus of the Hulun Buir Plateau and the plague epidemic in the M. unguiculatus plague focus of the Inner Mongolian Plateau, which greatly increases the difficulty of local prevention and control.
To prevent and control the resurgence of plague in the M. sibirica plague focus of the Hulun Buir plateau, it seems necessary to do health education on forbidding the flaying and eating of marmots. In the natural plague foci of marmots in China and Mongolia, the plague in humans is mainly contracted through wounds when flaying and eating infected marmots (30–31). Few cases have been caused by the consumption of raw marmot meat, which were eventually followed by septicemic plague or pneumonic plague (32). Unlike the marmot plague foci, human cases in other plague natural foci were mostly caused by fleabites (22). However, it should be noted that in the vast grazing region, due to special methods of food storage, infected rats may occasionally directly contaminate food, and domestic cats might also contaminate food (dried meat, cheese, etc.) by eating infected rats together with food stored by herdsmen. Then, herdsmen eating contaminated raw food could cause enteric plague. For example, the first case of pneumonic plague among herdsmen in Inner Mongolia in 2019 is likely to have developed from enteric plague because the earliest symptoms of this case occurred acutely in the abdomen (16). Therefore, in M. unguiculatus plague foci, attention should be paid to not only the carriage status in fleas but also the possibility of plague spreading through the digestive tract. In the M. unguiculatus plague focus of the Inner Mongolian Plateau, in addition to the traditional dissemination of health information about plague prevention, it is also necessary to promote knowledge about healthy eating habits among herdsmen.
Our study addressed the epidemic and distribution characteristics both of human and plague in animals from 1950 to 2019. We found that the plague occurring in the M. unguiculatus plague focus of the Inner Mongolian was sporadic. In recent years, cases have been gradually confined to the four neighboring counties, and reported cases are on the rise. It is necessary to be vigilant against possible outbreaks of plague in humans in this epidemic area. In addition, we predict that the trend of rodent density will first rise and then fall and be a prerequisite for the occurrence of plague in humans in the M. unguiculatus plague focus of the Inner Mongolian Plateau. The threat of plague resurgence in the M. sibirica plague focus of the Hulun Buir Plateau, coupled with the sporadic plague that occurred in the M. unguiculatus plague focus of the Inner Mongolian Plateau, has posed a new challenge to the control of plague in Inner Mongolia.
Funding: This work was supported by National Major Science and Technology Projects of China (2018ZX10713-003-002, 2018ZX10713-001-002).
Acknowledgment: American Journal Experts (Sub ID YNR9ZR12).
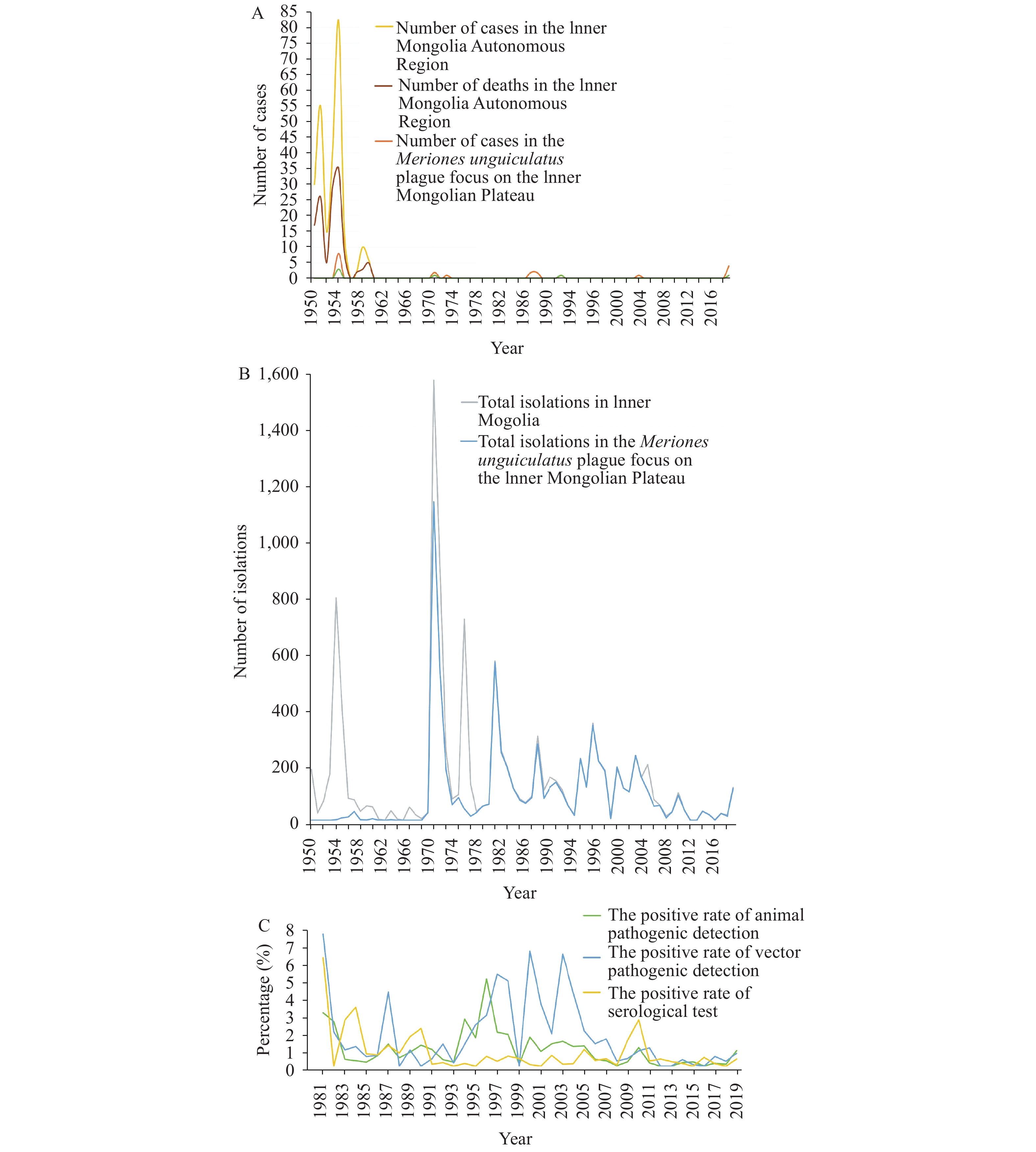 Figure 1.
Figure 1.Distribution over time of plague in humans and animals in the Inner Mongolian Autonomous Region.
(A) Distribution over time of plague in humans in the Inner Mongolian Autonomous Region from 1950 to 2019. (B) Distribution characteristics of Yersinia pestis isolates detected in the Inner Mongolia Autonomous Region from 1950 to 2019. (C) Distribution characteristics of pathogenic and serological detection results of plague hosts in the Inner Mongolia Autonomous Region from 1981 to 2019. Figure 2.
Figure 2.Spatial distribution of the Meriones unguiculatus plague focus on the Inner Mongolian Plateau from 1950 to 2019.
Year County Number of cases 1954 Hangjinhou 8 1970 Sunitezuo 1 1970 Wulatezhong 1 1972 Shangdu 1 1986 Wulateqian 2 1987 Etuokeqian 2 1991 Siziwang 1 2004 Suniteyou 1 2019 Sunitezuo 2 2019 Siziwang 1 2019 Xianghuang 1 Table 1. Distribution of plague cases in humans in the natural plague focus of Meriones unguiculatus on the Inner Mongolian Plateau from 1950 to 2019.
Item The third stage (1981–1999) The fourth stage (1999–2019) Total (1981–2019) Investigation area of M. unguiculatus (ha) 20,788.95 26,533.00 47,321.95 Density of M. Unguiculatus (/ha) 4.50 3.49 3.93 Nocturnal rodent capture rate (%) 4.55 3.43 3.98 Percentage of hosts infested with fleas (%) 23.04 27.83 25.56 The host-flea index 0.57 0.76 0.67 Percentage of the passage of burrows infested with nomadic fleas (%) 2.39 11.53 6.96 The index of nomadic fleas at the passage of burrows 0.09 0.24 0.16 Percentage of nests infested with fleas (%) 55.79 59.81 57.91 The nest-flea index 8.40 8.22 8.31 M. Unguiculatus positive rate (%) 1.15 0.55 0.87 Flea positive rate (%) 2.15 1.61 1.88 Indirect hemagglutination assay positive rate of M. unguiculatus (%) 1.39 0.42 1.06 Table 2. The epidemiological characteristics of host (Meriones unguiculatus) and vector surveillance in the Inner Mongolian Autonomous Region from 1981 to 2019.
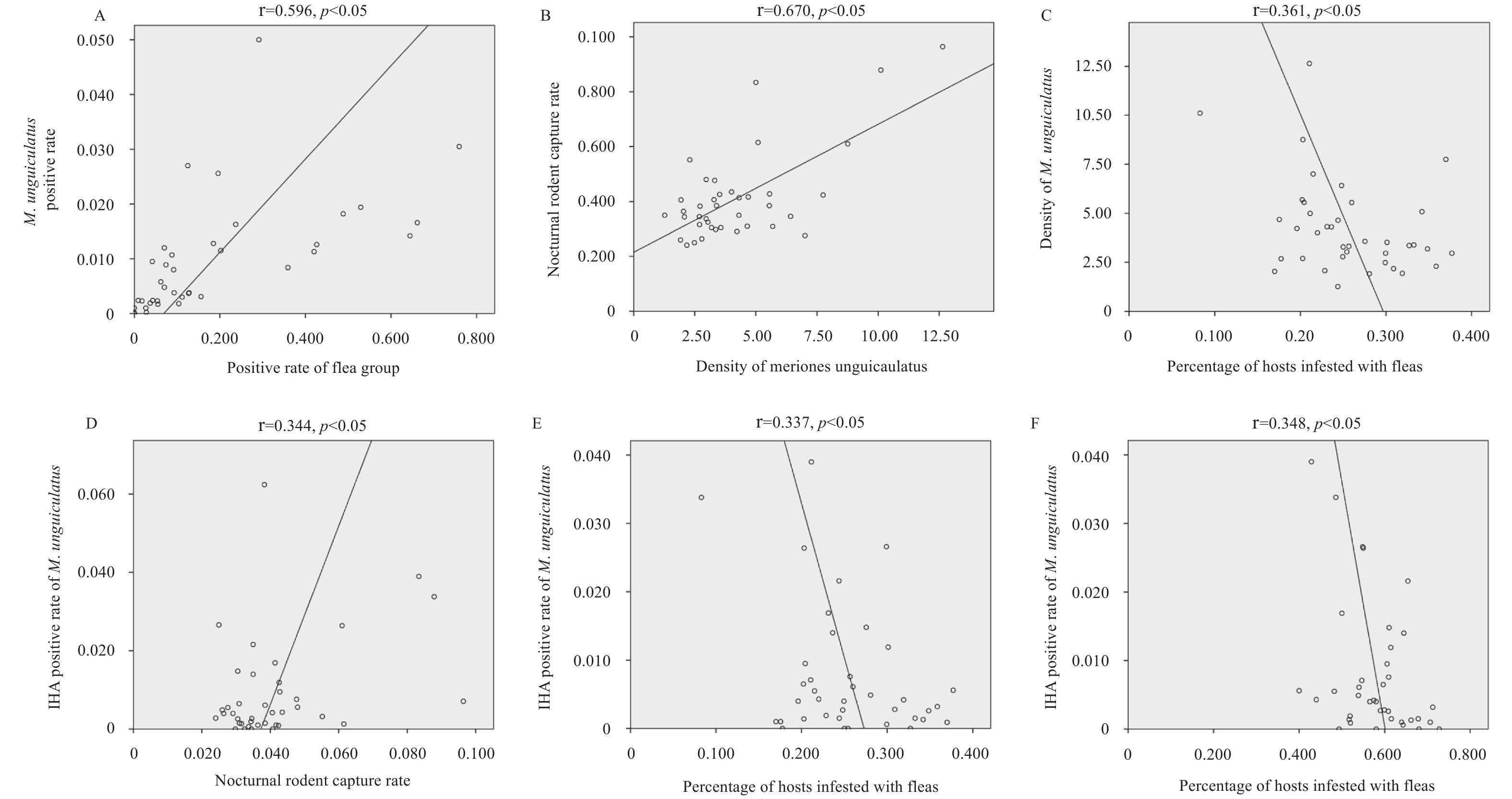 Figure 3.
Figure 3.Correlation analysis scatter plot and chi-squared test results of plague-related factors among animals in the natural plague focus of Meriones unguiculatus (M. unguiculatus) on the Inner Mongolian Plateau.
(A) The positive rate of M. unguiculatus and the positive rate of the flea group. (B) Density of M. unguiculatus and the nocturnal rodent capture rate. (C) Density of M. unguiculatus and the percentage of hosts infested with fleas. (D) IHA positive rate of M. unguiculatus and the nocturnal rodent capture rate. (E) Indirect Hemagglutination Assay (IHA) positive rate of M. unguiculatus and the percentage of hosts infested with fleas. (F) IHA positive rate of M. unguiculatus and the percentage of nests infested with fleas.Factor b χ2 p Intercept 0.140 64.717 <0.000 Density of M. unguiculatus (/ha) –0.001 3.237 0.072 Nocturnal rodent capture rate 0.076 0.628 0.428 Percentage of hosts infested with fleas (%) –0.019 0.771 0.380 The host-flea index –0.011 5.394 0.020 Percentage of nests infested with fleas (%) –0.241 46.991 <0.000 The nest-flea index 0.003 46.320 <0.000 Percentage of the passage of burrows infested with nomadic fleas (%) –0.192 11.680 0.001 The index of nomadic fleas at the passage of burrows 0.055 4.576 0.032 Table 3. Generalized linear model parameters of the indirect hemagglutination assay (IHA) positive rate of Meriones unguiculatus (M. unguiculatus) in the plague natural focus of M. unguiculatus on the Inner Mongolian Plateau.
HTML
Epidemiological Characteristics of Plague Cases in Humans in the Inner Mongolia Autonomous Region
Plague Surveillance Overview Among Animals in the Inner Mongolia Autonomous Region
Study of Factors Related to Plague Among M. unguiculatus in the M. unguiculatus Plague Focus of the Inner Mongolian Plateau
| Citation: |



 Download:
Download:




