-
The coverage and quality of screening are essential for reducing the incidence of cervical precancers and cervical cancer. This multicenter study aimed to investigate the relationship between screening quality and the detection of cervical precancers and cervical cancer. The study was conducted from June to December 2021 in six hospitals across six provinces. The 2,945 participants were non-pregnant women who underwent colposcopy examinations. The average age of participants was 40.9±11.5 years old. Only 6.9% of participants had received human papillomavirus (HPV) vaccination. A total of 92.6% of participants had abnormal cervical screening results. Of the participants, 577 had high-grade squamous intraepithelial lesions (HSIL) or worse (≥HSIL), with a detection rate of 19.6%. Univariate analysis indicated that a lack of cervical cancer screening history in the past five years, as well as positive cervical screening and abnormal colposcopic impression, were independent associated factors of the ≥HSIL detection rate. A multivariable logistic regression showed that positive cervical screening [odds ratio (OR) =1.75, 95% confidence interval (CI): 1.07–2.86] was a risk factor for detecting ≥HSIL. Low-grade, high-grade, and cancer of colposcopic impression were associated with a higher risk for detecting ≥HSIL (OR=2.94, 95% CI: 2.13–4.08; OR=36.64, 95% CI: 26.07–51.48). It is important to disseminate health knowledge to improve public awareness of cervical cancer prevention and to enhance the capacity building of professional staff to improve the quality of cervical cancer screening.
Cervical cancer was the second most common cancer and the second leading cause of cancer-related death among women of reproductive age worldwide in 2020, with 604,127 new cases and 341,831 deaths (1). Of these, 88.1% of the new cases and 91.4% of the deaths occurred in low- and middle-income countries. In 2016, China reported 98,900 new cases and 30,500 deaths due to cervical cancer (2). Cervical cancer can be prevented through vaccination and screening with appropriate follow-up and treatment (3). Early detection and treatment of cervical precancers are also key to successful prevention. Cervical precancers include HSIL and adenocarcinoma in situ (AIS). Cervical cancer screening, colposcopy, and pathology are three steps for diagnosing cervical precancers. Therefore, the coverage and quality of screening are essential to reducing the incidence of cervical precancers. The objective of this multicenter study was to investigate the relationship between screening quality and the detection of cervical precancers and cervical cancer in hospitals, providing evidence to improve cervical control for the target population in health facilities.
The study sites were six hospitals in six provincial-level administrative divisions (PLADs): Peking University First Hospital, Sichuan Provincial Maternity and Child Health Care Hospital, Women’s Hospital School of Medicine Zhejiang University, Yanbian Maternal and Child Health Care Hospital, Changzhou Maternal and Child Health Care Hospital, and Maternal and Child Health Hospital of Guangxi Zhuang Autonomous Region. The participants were all non-pregnant women who underwent colposcopy examination in the study hospitals from June to December 2021. A biopsy was performed after colposcopy examination, followed by biopsy specimens for pathological diagnosis. The collected data included age, cervical cancer screening history, HPV vaccination, cervical cancer screening, colposcopy examination, and pathology results. All the colposcopy doctors in the six hospitals had received training in colposcopy operations. The study was approved by the Biomedical Research Ethics Committee of Peking University First Hospital, with the ethic code 2020[321]. Finally, among 3,637 women, 2,945 participants were analyzed. The exclusion criteria included incomplete data collection. The outcome of this study was the detection of cervical precancers and cervical cancer (≥HSIL). SPSS software (version 26.0, IBM, Armonk, NY, USA) was used for statistical analyses, and P<0.05 was considered a statistically significant difference. Continuous variables were expressed as mean±standard deviation (SD); categorical variables were expressed as numbers and percentages; comparisons among groups were performed by Chi-square tests or Fisher exact tests as appropriate. Multivariable logistic regression models were used to evaluate the association between risk factors and detection of ≥HSIL.
The average age of participants was 40.9±11.5, ranging from 19 to 80 years old; 17.4% (511/2,945) were ≤29 years, 34.1% (1,003/2,945) were 30–39 years, 23.6% (696/2,945) were 40–49 years, 18.2% (536/2,945) were 50–59 years, and 6.8% (199/2,945) were ≥60 years. Only 6.9% (204/2,945) of participants had received HPV vaccination, with 4.3% (127/2,945) having completed the three-dose regimen. Additionally, 50% (1,473/2,945) of participants had a cervical cancer screening history within the past five years.
Among 2,945 participants, 80.6% (2,373/2,945) received HPV combined cytology co-testing screening, while 19.4% (572/2,945) received cytology screening. Overall, 92.6% had abnormal cervical screening results, including cytology ≥atypical squamous cells of undetermined significance (ASC-US) or HPV positive, or both abnormal cytology results and HPV positive. Only 7.4% underwent colposcopic examinations for abnormal symptoms. According to the colposcopic impression, 34.1% (1,004/2,945) of participants were normal/benign, 48.4% (1,426/2,945) were low-grade, 16.0% (470/2,945) were high- grade, and 1.5% (45/2,945) were cancer. Through biopsy and pathology diagnosis, 80.4% (2,368/2,945) were ≤low-grade squamous intraepithelial lesions (LSIL); 537 participants were HSIL, and the percentage was 18.2%; 13 participants were AIS, and the percentage was 0.4%. Additionally, 27 women (0.9%) were diagnosed with invasive cervical cancer (ICC). The participants with ≥HSIL totaled 577, and the detection rate was 19.6%. Of these 577 participants with ≥HSIL, 471 (81.6%) had HPV combined cytology co-test for cervical cancer screening and 106 (18.4%) had cytology tests for cervical cancer screening (Figure 1).
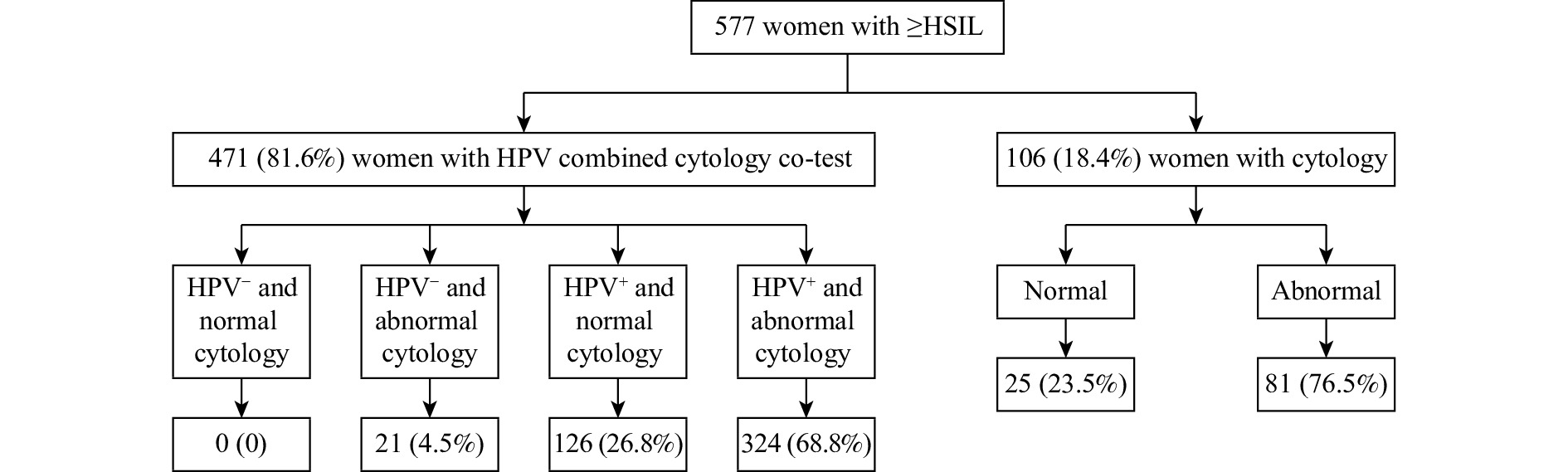 Figure 1.
Figure 1.Distribution of ≥HSIL with different screening methods (n, %) — six provinces, China, June–December 2021.
Abbreviation: HSIL=high-grade squamous intraepithelial lesion; HPV=human papillomavirus.The results of Table 1 indicated that a lack of cervical cancer screening history within the past 5 years, as well as a positive cervical screening and an abnormal colposcopic impression, were independent associated factors of ≥HSIL detection. The results of multivariable logistic regression to evaluate the associations between clinical risk factors and ≥HSIL are presented in Table 2. Positive cervical screening was found to be a risk factor for detecting ≥HSIL (OR=1.75, 95% CI: 1.07–2.86). Additionally, low-grade, high-grade, and cancer of colposcopic impression were associated with a higher risk for detecting ≥HSIL (OR=2.94, 95% CI: 2.13–4.08; OR=36.64, 95% CI: 26.07–51.48).
Clinical risk factor ≤LSIL*, n (%) ≥HSIL*, n (%) χ2 P Age (years) 5.38 >0.05 ≤29 405 (79.3) 106 (20.7) 30–39 792 (79.0) 215 (21.0) 40–49 572 (82.2) 124 (17.8) 50–59 443 (82.6) 93 (17.4) ≥60 156 (77.9) 43 (22.1) HPV vaccination 4.93 >0.05 Yes 163 (79.9) 41 (20.1) No 2,205 (80.4) 536 (19.6) Cervical cancer screening history in 5 years 32.82 <0.001 Yes 1,137 (82.7) 217 (17.3) No 1,158 (78.1) 320 (21.9) Unknown 73 (64.5) 40 (35.4) Cervical cancer screening method 0.51 >0.05 Cytology 466 (81.5) 106 (18.5) HPV combined cytology co-test 1,902 (80.2) 471 (19.8) Cervical cancer screening results this time 13.10 <0.001 Abnormal 2,018(79.4) 525 (20.6) Normal 350(87.1) 52 (12.9) Colposcopic impression† 759.68 <0.001 Normal/benign 953 (94.9) 51 (5.1) Low-grade 1,237 (86.7) 189 (13.3) High-grade 177 (37.7) 293 (62.3) Cancer 1 (2.2) 44 (97.8) Abbreviation: HSIL=high-grade squamous intraepithelial lesion; LSIL=low grade squamous intraepithelial lesion; HPV=human papillomavirus.
* ≤LSIL includes normal cervix and LSIL; ≥HSIL included HSIL and ICC.
† The Fisher exact test was used due to the small sample size of one cell (<5).Table 1. Characteristics of participants by detection of ≥HSIL (n, %) — six provinces, China, June–December 2021.
Risk factor Detection of ≥HSIL OR* 95% CI P Cervical cancer screening results Normal Ref. Abnormal 1.75 1.07–2.86 <0.050 Colposcopic impression Normal/benign Ref. Low-grade 2.94 2.13–4.08 <0.001 High-grade and malignant cancers 36.64 26.07–51.48 <0.001 Abbreviation: HSIL=high-grade squamous intraepithelial lesion; CI=confidence interval; OR=odds ratio.
* Adjusted for age, HPV vaccination, and cervical cancer screening history in the past 5 years.Table 2. Multiple logistic regression analysis of related clinical factors on detection of ≥HSIL — six provinces, China, June–December 2021.
-
In our study, the detection rate of ≥HSIL in six hospitals was 19.6%. Having no cervical cancer screening history in the past five years, a positive cervical screening, and an abnormal colposcopic impression were identified as clinical risk factors for detecting ≥HSIL.
Our study showed that the detection rate of ≥HSIL was 19.6%, which is similar to the results of other studies. A study from Chongqing Hospital reported that among 1,055 participants, 211 cases were ≥cervical intraepithelial neoplasia 2 (CIN2), resulting in a detection rate of 20% (4). Previous studies have reported that the detection of CIN and ICC ranged from 4.1 to 6.0 per 1,000 in different age groups with different cervical cancer screening methods in the general population (5). In hospitals, most patients had abnormal cervical screening results, making them a high-risk population, thus the detection rate of cervical cancer was much higher than in the general population.
Our study revealed that a lack of cervical cancer screening within the past 5 years and abnormal screening results are associated with the detection of ≥HSIL. Specifically, abnormal screening results were found to increase the risk of ≥HSIL detection by 75% compared to normal screening results. Cervical cancer screening history was a risk factor for advanced stages of cervical cancer; a study from Denmark found that the less advanced invasive cervical cancer stage (stage I) was 3.14 times higher given adequate attendance to cervical cancer screening programs, with 61.6% of patients having deficient screening histories (6). An American research also indicated that 60% of women aged 21 years and older who were diagnosed with invasive cervical cancer had no cervical cancer history among 367 participants (7). Cervical cancer screening programs have been conducted by the Chinese government since 2009, but only half of the participants attended screening in the last 5 years in our study. The percentage of deficient screening histories in our study was slightly lower than those of previous studies (6-7), which may be related to the different age range and observation indicators. The study reported that abnormal cervical cancer screening results were a risk factor for the detection rates of HSIL and ICC (OR=1.75). For a woman who has cervical cancer screening just once in her life after 35 years old, her risk of dying from cervical cancer would decrease by 70%. If she is screened every 5 years, her risk of dying from cervical cancer drops by more than 85% (8). Therefore, cervical cancer screening is an efficient secondary preventable measure; it is not only about screening on time, but also a long-term management strategy for controlling cervical precancer and cancer. According to the report about cervical cancer screening coverage estimates of 202 countries, 1.6 billion (67%) of 2.3 billion women aged 20–70 years had never been screened for cervical cancer (9). In China, the population-based screening rate is lower (10). It is necessary to conduct more extensive health education to enhance awareness of cervical cancer among the target population, and then to increase the initiative screening rate.
Colposcopy examination is a key method for diagnosing cervical precancer as a second step, and it requires a more professional and trained doctor. According to the quality control manual of cervical screening, the high-grade coincidence rate between colposcopy impression and biopsy should be ≥60% (11). The coincidence rate in our study was 62.3% (HSIL), meeting the quality control requirement. The doctors in the six study hospitals had received training, which enabled them to provide high-quality colposcopy examinations and improve the detection rate of cervical precancer.
HPV vaccination is an important primary prevention method; however, due to the low vaccine coverage rate, it has not been a significant factor in our study. Most women were screened by HPV combined cytology co-test, but the detection rate of cervical precancers did not differ significantly between different screening methods. The detection rate was mainly influenced by the quality of colposcopy and the screening results. There were several limitations to our study. Firstly, we focused mainly on clinical factors affecting the detection rate of cervical precancers in medical facilities, not including social factors. Secondly, most participants underwent colposcopy examination due to abnormal cervical cancer screening results, and few participants underwent colposcopy examination for abnormal symptoms; however, we did not collect more detailed information on abnormal symptoms. Finally, in this study, we only collected data from participants who underwent colposcopy examination, and the cervical cancer screening results may have come from different health facilities or different screening methods, so we could not compare the relationship between screening quality and detection of ≥HSIL. Therefore, further research with larger populations, higher quality, and multiple sites is needed in the future.
The World Health Organization's cervical cancer elimination campaign must increase both screening coverage and treatment of detected cervical precancers (9). Our study indicated that cervical cancer screening and colposcopy impression were the main clinical influencing factors in health facilities. To achieve elimination goals, it is essential to disseminate health knowledge to improve public awareness of cervical cancer prevention and to enhance capacity building of professional staff to improve the quality of cervical cancer screening.
-
All the participants and investigators from 6 hospitals.
-
The authors do not have any competing interests.
HTML
| Citation: |




 Download:
Download:




