-
After nearly 40 years of research and control efforts, much progress has been made to accomplish the most significant achievements in antiviral drug development and cocktail therapy that has transformed the human immunodeficiency virus/acquired immunodeficiency syndrome (HIV/AIDS) treatment regiment from having the highest mortality into a manageable chronic disease like hypertension or diabetes. The control goals for AIDS established by the World Health Organization (WHO) and the Joint United Nations Programme on HIV/AIDS (UNAIDS) set a target of treating 30 million HIV/AIDS patients by the end of 2020 (1). However, more work is needed to achieve the prevention goal set at 2020 of having less than a half million new HIV infections since there were still 1.7 million new HIV infections by the end of 2019 (2).
Close to 80 million people have been estimated to be infected by HIV since it was first detected in 1981, and roughly half have died. The global AIDS pandemic has been characterized by a fast mutating virus [at least 10 times faster than the coronavirus disease 2019 (COVID-19) virus] that has a relatively long incubation time (7 to 8 years for AIDS compared to 7 to 14 days for COVID-19) and largely heterogenous distribution amongst various countries (from over 30% to less than 0.1%) due to the diversity of human behaviors. There is no silver bullet to control HIV/AIDS and comprehensive strategy and measures are always needed. However, it is believed that an effective HIV vaccine will provide a useful tool to achieve full control of HIV/AIDS.
All conventional vaccine procedures and modern techniques have been applied to HIV vaccine research in the past 3 decades. After over 300 HIV vaccine clinical trials have been conducted, no vaccine candidate nor clear path to success remains in sight. Historically, the major hurdle in vaccine development has not been making the vaccine itself, but rather finding the right pathogen causing the disease, especially for emerging diseases, since traditional vaccines can be produced by following traditional vaccine procedures of killing or attenuating the original pathogens or taking the genetic vaccine procedure of using the immunogens and getting rid of the toxic parts of the pathogen.
However, the above conventional vaccine technologies work well only for the diseases of category A pathogens, but not for category B pathogens, a term I named. The major difference between the two rely on if the pathogen can induce protective immunity in humans during natural infection (category A) or not (category B). The key to success for category A vaccines are due to evolution, in which the human immune system is strong enough to control the pathogen as demonstrated by many asymptomatic infections and self-limiting disease courses such as in hepatitis B virus or even COVID-19 virus infections (Figure 1) (3). Therefore, category A vaccines are nature-born vaccines and gifts from evolution. The most rapidly made category A vaccine is the swine influenza vaccine in 2009. It only took the Chinese pharmaceutical companies less than 100 days from receiving the virus strains from the WHO to manufacturing the vaccine, conducting clinical trials, and receiving Chinese Food and Drug Administration (China FDA) licensing to administer the vaccines to Chinese people to successfully control the 2009 H1N1 epidemic. The COVID-19 vaccine is another fast produced and licensed vaccine in 2020.
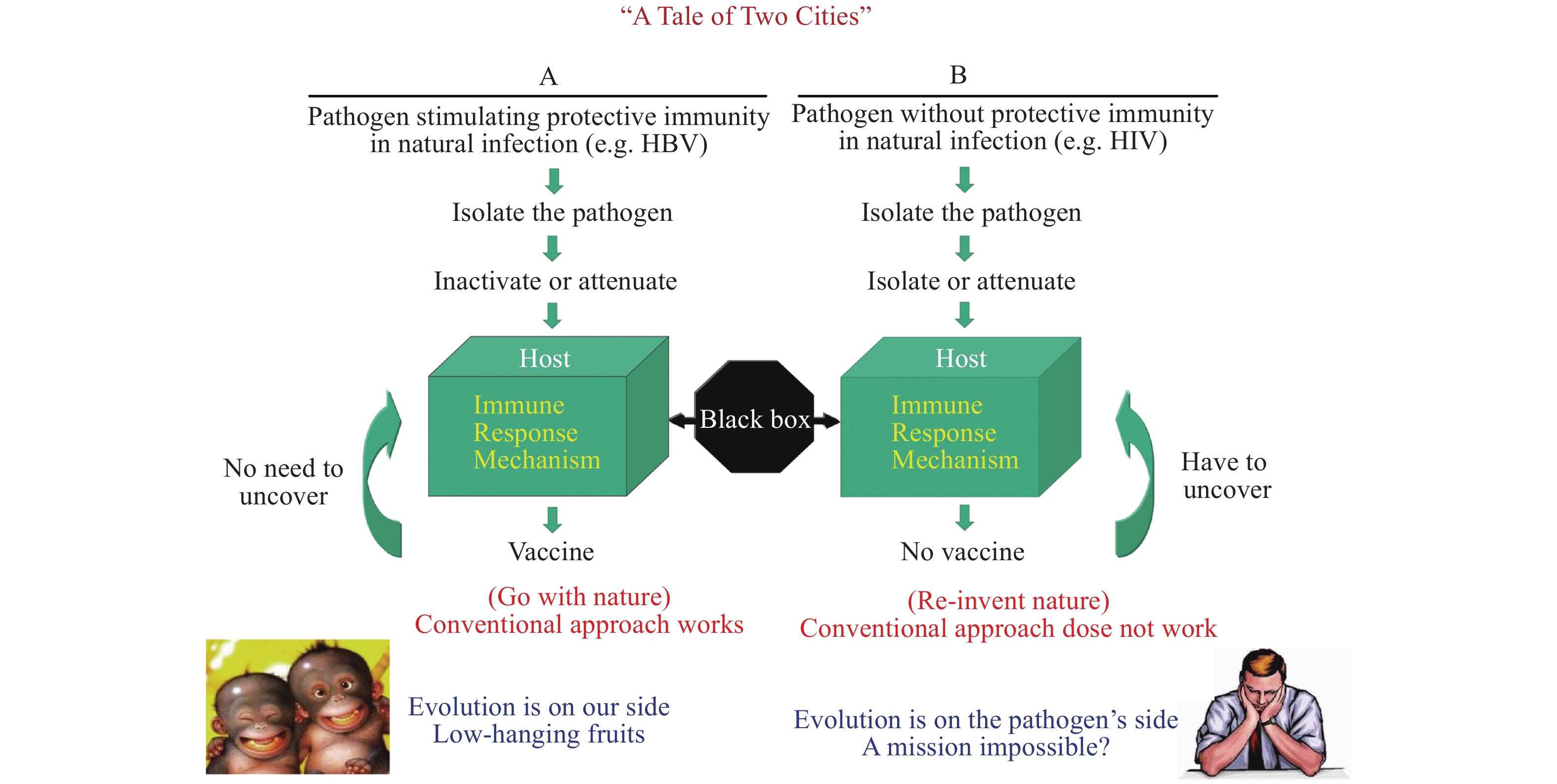 Figure 1.
Figure 1.Different research designs and approach for two vaccine typeesigns and approach for two vaccine types.
Unfortunately, the HIV pathogen falls into category B, which do not induce enough protective immunity in the course of human natural infection. So far, not a single virus-free sera convertor with HIV has ever been found. The biggest barrier to a successful HIV vaccine is to empower the ineffective human immune system to evolve to defend us against HIV infection. While there is no need for sophisticated vaccine design or to understand the mechanism of immune protection against category A pathogen infection, we must discover novel approaches other than the conventional vaccines procedures to catch up with evolution.
Therefore, HIV vaccine research at the China CDC is based on the hypothesis that one can only induce protective immunity by either redesigning the HIV immunogen or modulating the host immune response to control the HIV infection. To test our hypothesis, we developed 4 working strategies: 1) systematically screening the circulating HIV-1 strains to identify the predominant vaccine strain (4–5); 2) learning for protective immunity from the world’s first lentivirus vaccine (HIV belong to lentiviruses), the equine infectious anemia virus (EIAV) vaccine, to shed light how to design an HIV vaccine (6–7); 3) improving HIV vaccine immunogenicity by redesigning conserved regions and utilizing novel immunization protocols (8); and 4) using replication competent viral vectors to stimulate balanced immune responses with both antibody and T cell responses against HIV.
Following these strategies, we selected the most predominant HIV-1 clade, the CRF07 B’/C HIV-1 as the vaccine strain, which was independently confirmed as one of the best HIV vaccine candidates by US scientists (9). We chose the replication competent Tiantan vaccinia (TV), the Chinese smallpox vaccine strain, as the vaccine vector due to its strong immunogenicity and good safety records in smallpox eradication campaigns (10). In collaboration with scientists who developed the EIAV vaccine, we found that the broader T cells and neutralizing antibody (nAb) responses are related to the protection of the EIAV vaccine (11). The key amino acids in the conserved region of the envelope glycoprotein (env) of HIV-1 was genetically modified according to learning from the mutated env structure of the EIAV vaccine strain, aiming at improving its immunogenicity. The nAb induced by the modified HIV env can neutralize multiple strains from both HIV-1 B and B’/C viruses with broader neutralizing profiles compared with wide type HIV-1 env (Figure 2) (12).
 Figure 2.
Figure 2.The introduced mutations and improved immunogenicity of the modified HIV envelope based on the envelope structure of EIAV vaccine strain. A. Schematic envelope structure of the EIAV D510 and the HIV-1 CN54. The left figure shows the EIAV V3, V4 regions; the right figure shows the HIV-1 V1, V2 regions. N-Glycosylation sites are shown as branched lines. B. Comparative inhibition of HIV-1 infection by sera collected at Week 16 from mock-, gp145- and gp145-10M-immunized guinea pigs. The neutralizing experiment was conducted by using a panel of clinical HIV-1 isolates from PBMCs in TZM-bl cell assays. The dotted line in the figure indicates the 50% inhibition rate.
We developed both DNA and recombinant TV (rTV) vaccine candidates expressing HIV-1 CRF 07 gag, pol, and env genes in collaboration with China’s National Vaccine and Serum Institute (13–14). In preclinical testing, the two vaccines used in a priming (DNA vaccine) and boost (rTV) regiment could stimulate strong HIV1-specific cellular and humoral immune responses in small animals and monkeys. Those monkeys immunized with vaccine could resist infection when challenged with chimeric simian immunodeficiency virus (SHIV) containing homologous HIV1 gp120 (15).
After being approved by China FDA, we conducted Phase I clinical trials with DNA priming (at 0, 4, and 8 weeks) and rTV vaccine boost (at week 16) in 48 healthy volunteers in collaboration with Peking Union Medical College Hospital (ChiCTR-TRC-100128). The vaccines were well tolerated, and no severe adverse events were reported. Both antibody (100%) and T cell (over 60%) responses against HIV-1 were detected in subjects with DNA vaccine priming and rTV vaccine boost. The T cell responses in the vaccines were multifunctional (IL2/IFN-g/TNF-α) with stronger responses to CD4 cells than to CD8 cells (16).
We conducted the first Phase II clinical trial of the vaccines with priming boost regiment on 150 volunteers in collaboration with Beijing Youan Hospital. The vaccines were well tolerated with no serious adverse events (SAE) by all volunteers and again induced strong HIV-1 specific antibody (100%) responses and good T cell responses (over 60%). The vaccines could also induce good antibody to V1V2 loop but not much neutralizing antibody responses. To stimulate better neutralizing antibody responses, we discussed with the US National Institute of Allergy and Infectious Diseases (NIAID) and signed a memorandum of understanding (MOU) to conduct a Sino-US collaborative clinical trial of combining our DNA/rTV vaccines with the gp145 trimer vaccine in China (17).
While waiting for NIAID to finish the Phase I clinical trial of the gp145 vaccine in the US, we started the second Phase II DNA/rTV vaccine trial with a second rTV boost in order to stimulate stronger and longer lasting neutralizing antibody and T cell responses. The second Phase II clinical trial were conducted in 150 volunteers in two hospitals in Beijing and Hangzhou in a multicenter clinical trial format in preparation for future Phase IIb/III multicenter clinical trials of both the China CDC vaccines and the Sino-US joint vaccine trials. All vaccine injections were concluded in the second Phase II clinical trial. We are currently working closely with the HIV Vaccine Trials Network (HVTN) leadership group and NIAID to prepare the Investigational New Drug (IND) application to the China FDA for the joint Sino-US DNA/rTV/gp145 vaccine trials. We hope the joint Sino-US HIV vaccine clinical trial could be initiated in 2021 as an international collaborative effort to progress the global HIV vaccine programs.
HTML
| Citation: |




 Download:
Download:




