2021 Vol. 3, No. 21
What is known about this topic?
Few major outbreaks of coronavirus disease 2019 (COVID-19) have occurred in China after major non-pharmaceutical interventions and vaccines have been deployed and implemented. However, sporadic outbreaks that had high possibility to be linked to cold chain products were reported in several cities of China..
What is added by this report?
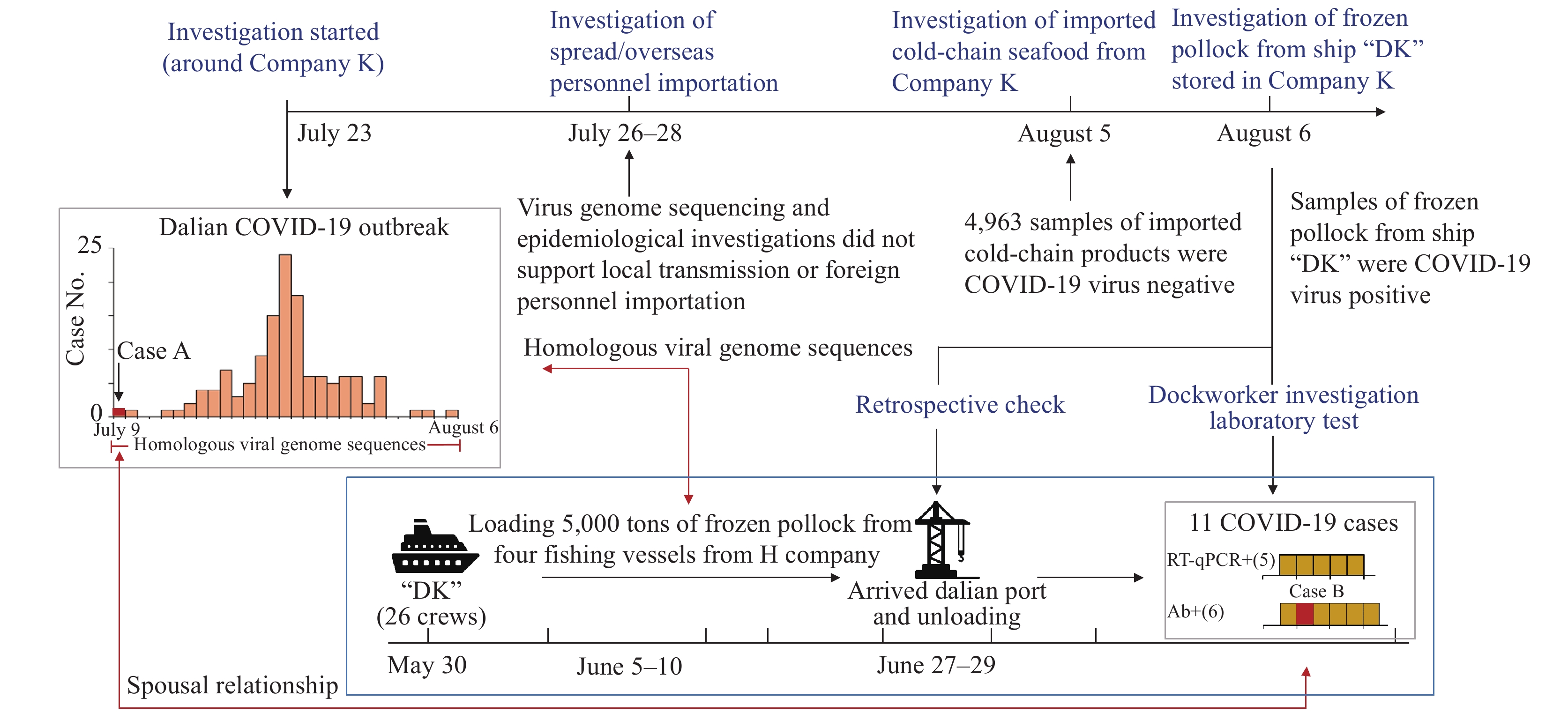
In July 2020, a COVID-19 outbreak occurred in Dalian, China. The investigations of this outbreak strongly suggested that the infection source was from COVID-19 virus-contaminated packaging of frozen seafood during inbound unloading personnel contact.
What are the implications for public health practice?
Virus contaminated paper surfaces could maintain infectivity for at least 17–24 days at -25 ℃. Exposure to COVID-19 virus-contaminated surfaces is a potential route for introducing the virus to a susceptible population. Countries with no domestic transmission of COVID-19 should consider introducing prevention strategies for both inbound travellers and imported goods. Several measures to prevent the introduction of the virus via cold-chain goods can be implemented.
Background: COVID-19 infection is a major public health problem worldwide, and the D614G mutation enhances the infectivity of COVID-19.
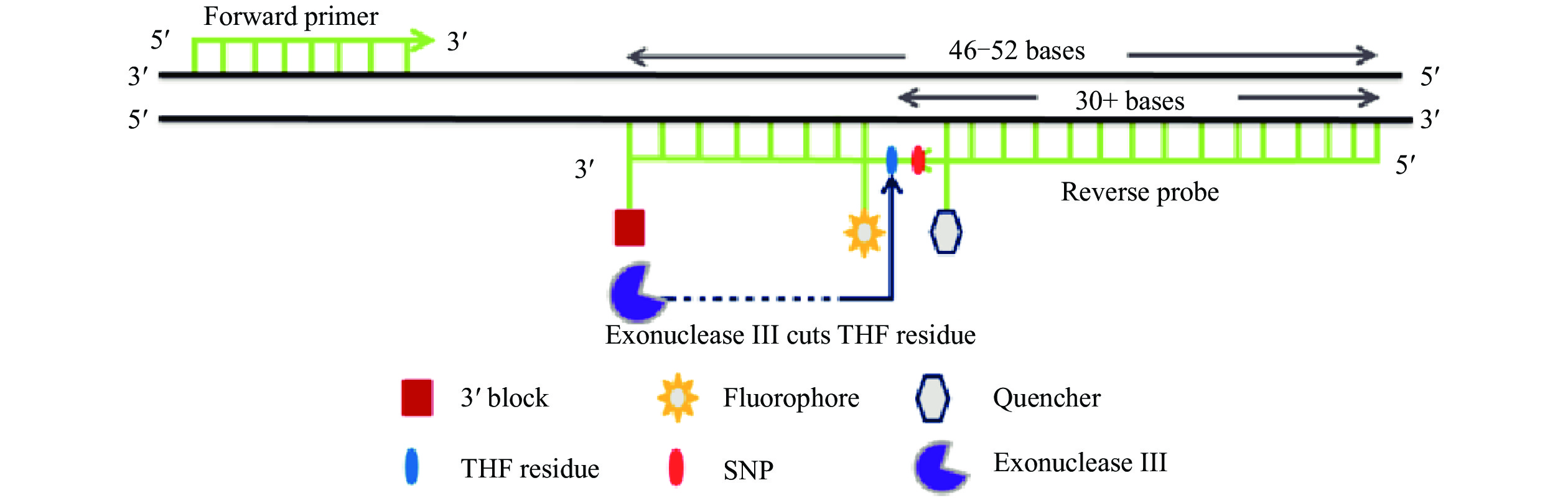
Methods: A probe-directed recombinase amplification (PDRA) assay was discussed to detect the D614G mutation at 39 ℃ for 30 min. The sensitivity, specificity, and reproducibility of the PDRA were evaluated by D614 and G614 recombinant plasmids. The clinical performance of PDRA assay was validated by testing of 53 previously confirmed COVID-19 positive RNAs and 10 negative samples. Direct sequencing was carried out in parallel for comparison.
Result: With good reproducibility and specificity, the PDRA assay worked well with the concentration in the range of 103–107 copies/reaction. Compared with direct sequencing as a reference, the recombinase-aided amplification (RAA) assay obtained 100% sensitivity and 100% specificity using clinical samples.
Conclusions: A rapid, convenient, sensitive, and specific method to detect D614G mutation was developed, which offers a useful tool to monitor mutations in COVID-19 virus RNA.



 Subscribe for E-mail Alerts
Subscribe for E-mail Alerts CCDC Weekly RSS Feed
CCDC Weekly RSS Feed